
[cmamad id=”23331″ align=”right” tabid=”display-desktop” mobid=”display-desktop” stg=””]
It’s true… Men can really get a thicker and longer penis without using pumps or pills. Here’s how…

—-Important Message from Our Sponsor—-
Proof of REAL growth “down there” – watch this proof video
If you’ve ever taken a girl home, gotten hot and heavy, and then felt embarrassment and PANIC when you take off your pants and see the look of DISAPPOINTMENT on her face…
You need to go check out this proof video right now…
What’s crazy about this is the ACTUAL VIDEO PROOF that this works…
…actual VIDEOS that can’t be faked…
I was 100% skeptical until I saw these videos… So even if you think it’s impossible to get bigger (and there’s no pills or suction devices or any of that crap)…
…go check out the overwhelming proof right here in this video.
———-
Folic acid – good or bad for men?
Folic acid is added to almost EVERY food.
But that’s too bad.
Because many men are eating the wrong form of folic acid.
And the consequences can be dire.
This article explains how to avoid the bad forms of folic acid, and how to get the amount that will promote good mind, body, and sexual health.
Let’s start with this fact:
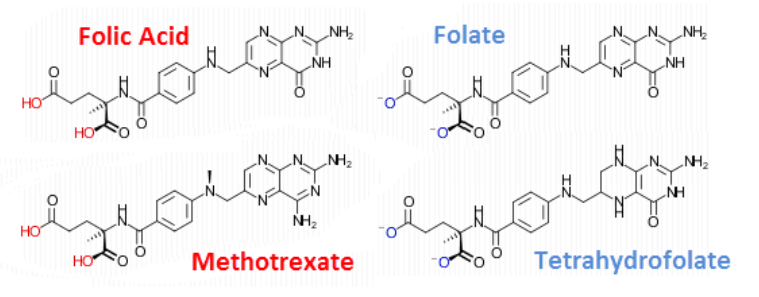
Folic acid is NOT folate.
And you need folate, but you should NOT eat foods containing folic acid.
Not only is the wrong folate completely inactive in the enzymes that require natural forms (i.e. tetrahydrofolate, methylenetetrahydrofolate, or 5-formyl- tetrahydrofolate)…
It also actually inhibits these enzymes.
This means that too much folate can reduce DNA synthesis and brain myelination – and induce lipid peroxidation via homocysteine.
(Myelination has to do with the functioning of our nervous system, homocysteine is an amino acid we get from eating meat, and lipid peroxidation is a form of cell damage.)
There is also some confusion surrounding the nomenclature: Many online articles set up a “folic acid vs folate” dichotomy – yet these two become equivalent at the same pH.
The moment dry folic acid crystals are mixed with water, or added to many foods, they immediately become folate.
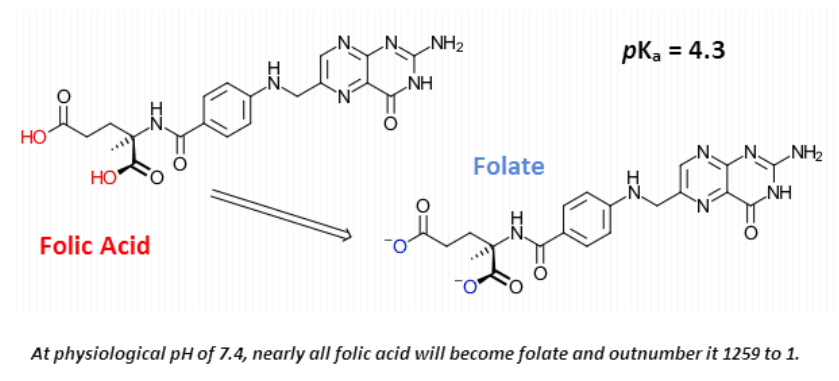
These are readily interconvertible in solution.
The important difference is the number of electrons in the ring…
The biologically active folates all have more electrons in the ring and are considered electrically “reduced” for this reason.
Alternatively, these folates can be seen as “hydrogenated.” The extra electrons in the pteridine ring can form additional bonds – usually to hydrogen.
[cmamad id=”23332″ align=”center” tabid=”display-desktop” mobid=”display-desktop” stg=””]
The “acid” part of the term “folic acid” refers to the attached glutamic acid… Yet they all have this.
We can make a far more useful distinction between folate, dihydrofolate, and tetrahydrofolate:
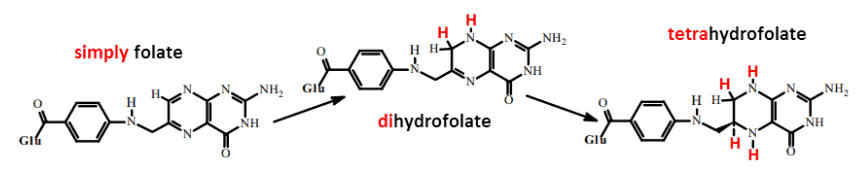
While it’s true that a certain amount of folate is converted into the active form (in the liver), there is a limit to this.
Anything we ingest above and beyond what our liver can transform becomes an officially recognized enzyme inhibitor.
And this can lead to a reduction in DNA replication and in central nervous system sphingomyelin synthesis.
(Sphingomyelin is stuff that helps our cells communicate and helps them decide when to die, among other things.)
In other words, that situation is unhelpful to many of our important enzymes.
And this study here shows us why:

The enzyme responsible for reducing dihydrofolate is, perhaps not surprisingly, called dihydrofolate reductase.
Yet, this enzyme also reduces folate: first to dihydrofolate and then again to the active tetrahydrofolate.
The only two places this enzyme exists are in the liver and in the placenta.
This enzyme is not present in the brain.
Any folate that crosses the blood-brain barrier, as such, will compete with the tetrahydrofolates and actually inhibit essential enzymes.
This group of scientists made some important findings…
Check out this graph. You can see that, compared to rats, humans have relatively low activity of dihydrofolate reductase:
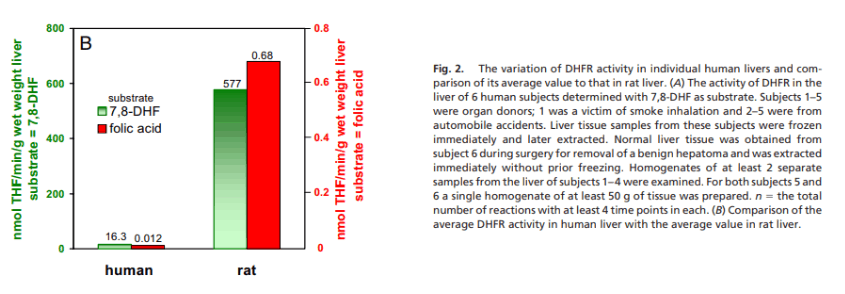
Also notable is the fact that rats can transform both folate and dihydrofolate to roughly the same extent…
On the other hand, humans can barely reduce folate at all.
The rate of dihydrofolate to tetrahydrofolate conversion in humans ranges between 10 to 25 nanomoles per minute per gram, while that of folate to tetrahydrofolate is .02 nmol/min/g.
So, not only do our enzymes have completely pathetic conversion rates (compared to rats no less), our folate ⟶ tetrahydrofolate conversion ability is barely even worth considering.
Our bodies weren’t forced to evolve enzymes to efficiently deal with folate because it only exists in ultra-trace amounts in nature, if at all.
Our liver appears to have evolved to deal only with the small amount of dihydrofolate that’s formed through DNA synthesis…
… and not this new food-folate that needs to be reduced twice.
This is a huge metabolic burden.
In addition, it happens so slowly (relative to dihydrofolate) that it’s considered to significantly inhibit normal reactions.
So this is the deal: The synthetic food-folate known as “folic acid” is an official enzyme inhibitor or an antifolate drug:
And this is why it can be toxic in large amounts – even though it can be beneficial for malnourished people, in smaller doses.
Antifolate drugs are sometimes used during chemotherapy for cancer – to inhibit DNA synthesis.
And those are not much different from folic acid.
Methotrexate is the most common:
Plasma levels are important because the high-affinity folate receptors of the blood-brain barrier actually have a higher affinity for folate.
“The affinity for the folate transport system is: folic acid > (±)-methyltetrahydrofolate = (+)-methyltetrahydrofolate > methotrexate.”
And since the brain does not have dihydrofolate reductase activity, any non-reduced folate that crosses the blood-brain barrier will inhibit important brain enzymes.
This perfectly explains its toxicity at high doses.
Non-reduced folate inhibits the following enzymes:
- Thymidylate synthase: This is necessary for the synthesis of deoxythymidine monophosphate, a nucleotide base. This is the rate-limiting enzyme of DNA synthesis.
- Thymidylate kinase: Adds the second phosphoryl group to thymidine phosphate, yielding thymidine diphosphate.
- Phosphoribosylaminoimidazolecarboxamide formyltransferase: This enzyme helps to synthesize the purine rings necessary for two other DNA bases, guanidine and adenosine, and uses 10-formyltetrahydrofolate.
- Serine hydroxymethyltransferase: This strips a one-carbon unit off of the serine backbone forming glycine and the 5,10-methylenetetrahydrofolate cofactor.
- Methylenetetrahydrofolate reductase: Reduces its eponymous compound to 5-methyltetrahydrofolate.
- Methionine synthase: The only enzyme that uses 5-methyltetrahydrofolate; this enzyme methylates homocysteine with the help of cobalamin (vitamin B12).
- Dihydrofolate reductase: Present in the liver and placenta yet not in the brain, this enzyme recycles dihydrofolates back into the active tetrahydrofolates.
It’s understandable that brain DNA synthesis should not be inhibited, so I’m not going to dig into that here.
But messing with the enzymes involved in the methyl cycle causes problems that are less obvious.
One-carbon methyl groups in the brain are essential; these go towards neurotransmitters and myelin formation.
Methyl groups (+CH3) are distributed onto homocysteine yielding methionine, a universal cofactor for countless enzymes:
+CH3 + homocysteine ⟶ methionine
(Remember, homocysteine is an amino acid in the blood. Methionine is another amino acid.)
The plasma and cerebrospinal fluid ratios of homocysteine to methionine are both very strong indicators of dementia, Down’s syndrome, and autism.
Familial hyperhomocysteinemia leads to low double-digit IQs. (Hyperhomocysteinemia = abnormally high levels of homocysteine in the blood.)
Besides the lack of brain methyl groups that a folate imbalance will lead to, homocysteine is, in itself, quite toxic:
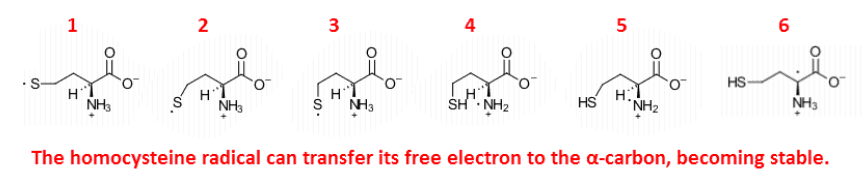
Homocysteine is unique in that it can form a stable lipid-soluble free radical, explaining the near perfect correlations between it and markers of lipid peroxidation.
A Pearson coefficient of 1 is a perfectly straight line, and so is the correlation between homocysteine and hydroxynonenal (a compound that’s associated with many diseases and a marker of oxidative stress):
“There were significant positive correlations between the cerebrospinal fluid concentrations of homocysteine and hydroxynonenal… There was also a significant positive correlation between the plasma concentration of homocysteine and the cerebrospinal fluid concentrations of homocysteine…”
Hydroxynonenal is a lipid peroxidation fragment. It doesn’t occur in any other way.
Besides perhaps being an indicator of more serious damage, hydroxynonenal and the very similar malondialdehyde are protein-crosslinking agents.
(Malondialdehyde is also a marker of oxidative stress.)
How much folic acid can our liver handle before non-reduced folate spills over into the blood and inhibits DNA-synthesizing enzymes?
Well, that’s about 400 micrograms via food and about 300 in freeform:

This was a simple experiment.
They recruited people and gave them folic acid either via food or supplements, using a variety of dose levels.
Then they checked changes in serum levels:
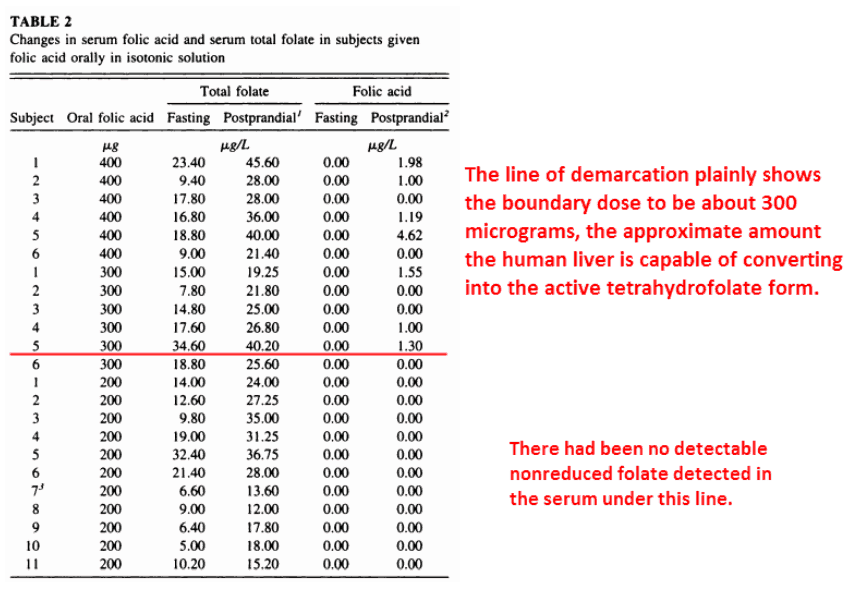
As you can see in the chart, 300 micrograms or under is completely safe, and could even be considered beneficial.
At this dose, folic acid will be:
(1) Deprotonated to folate in the stomach
(2) Reduced to, at least, dihydrofolate in the liver
(3) And eventually reduced again to essential tetrahydrofolate before entering circulation.
Yet many people surpass this dose.
Other studies indicate that about 21% of random people, post-fortification, had detectable levels of inhibitory non-reduced folate.
Since non-reduced folate inhibits enzymes and has a higher brain uptake than reduced folates, I think it would be good to stay under the 300-microgram cut-off point.
Reduced folates such as 5-methyltetrahydrofolate and folinic acid are widely available online…
They are helpful in instances where folic acid supplementation only exacerbates the problem, such as:
- Difficulty absorbing nutrients
- Alcoholism
- Pregnancy
- Bone marrow issues
Alternatively, just try to get enough folate from food. For example:
- Eggs
- Beans
- Leafy green vegetables such as spinach
—————–

- Kalmbach, R. "Circulating folic acid in plasma: relation to folic acid fortification." American journal of clinical nutrition (2008) https://www.researchgate.net/profile/Aron_Troen/publication/23245068_Circulating_folic_acid_in_plasma_Relation_to_folic_acid_fortification/links/02bfe50ef2781cad31000000/Circulating-folic-acid-in-plasma-Relation-to-folic-acid-fortification.pdf
- Kelly, P. "Unmetabolized folic acid in serum: acute studies in subjects consuming fortified food and supplements." The American journal of clinical nutrition (1997) https://www.ncbi.nlm.nih.gov/pubmed/9174474
- Spector, R. "Folate transport by the choroid plexus in vitro." Science (1975) https://www.jstor.org/stable/1739150
- Selley, M.. "The effect of increased concentrations of homocysteine on the concentration of (E)-4-hydroxy-2-nonenal in the plasma and cerebrospinal fluid of patients with Alzheimer’s disease." Neurobiology of aging (2002) https://www.ncbi.nlm.nih.gov/pubmed/11959400
- Bailey, S. "The extremely slow and variable activity of dihydrofolate reductase in human liver and its implications for high folic acid intake." Proceedings of the NAS (2009) http://williams.medicine.wisc.edu/folate_metabolism_variability.pdf
- Folic Acid | CDC https://www.cdc.gov/ncbddd/folicacid/about.html
- Folic Acid: MedlinePlus https://cybercemetery.unt.edu/archive/oilspill/20120915193739/http://www.nlm.nih.gov/medlineplus/folicacid.html
