
Wear it, use it, you can even inject it…
—-Important Message—-
Still avoiding the gym? This natural fat burner blasts away fat — no diet or exercise necessary

Bodybuilders are using it to cut weight…
Busy executives are using it to keep their energy levels high…
Husbands are using it to slim down and impress their wives in bed…
…and single men are using it to attract beautiful young women way out of their league…
It even works at home if you’re avoiding gyms right now.
Discover the natural fat burner making all diets and all gym memberships obsolete.
———-
This naturally kills of bad bacteria, may help prevent viruses
Silver has long been known to be the most effective element against bacteria.
That’s why silver is countless consumer products, medicines, and even fabrics.
But very few people know how silver can kill bacteria in parts per billion concentrations while completely leaving human cells alone.
Understanding the cellular mechanism of silver ions (Ag⁺) — and their production — means understanding one of the best antibiotics.
Silver ions (Ag⁺) are cheap to make, easy to produce, and are very small — they weigh 44% as much as penicillin and are even smaller in diameter.
Silver ions can diffuse into spaces where other treatments cannot.
‘Organisms (especially bacteria) show a low propensity to develop resistance to silver‐based products.’ ―Landsdown
And silver’s positive charge allows it to be transported inside the cell in a way similar to sodium (Na⁺) and potassium (K⁺) — attracted by the negative mitochondrial membrane potential.
And as an element: silver cannot be degraded and will always be effective.
When 1 bacterial cell is killed from silver, the Ag⁺ that is released can inhibit other bacteria with no loss of function.
‘…but rather the ability of bacteria, trypanosomes and yeasts to take up and concentrate silver from very dilute solutions. Therefore, bacteria killed by silver may contain 10⁵–10⁷ Ag⁺ per cell…’ ―Landsdown
So silver really has some very attractive properties for an antibiotic.
The second most common elemental antibiotic — iodine (I₂) — becomes inactivated the moment it acts, forming new and irreversible chemical bonds with the bacterial lipids.
So most antibiotics are one-shot deals, but silver is persistent.
And silver ions are easy to acquire, safe, and can even be made cheaply at home.
So how is this high selectivity explained? How does silver kill bacteria more than any other element, while also being practically the safest uncommon element in the human body?
‘Silver is the most toxic element to microorganisms in the following sequence: Ag > Hg > Cu > Cd > Cr > Pb > Co > Au > Zn > Fe > Mn > Mo > Sn.’ ―Zhao
Silver binds to DNA, an observation made since the ‘60s:

This binding effect is very specific: silver binds to DNA more than any other metal ion, even more than mercury and copper.
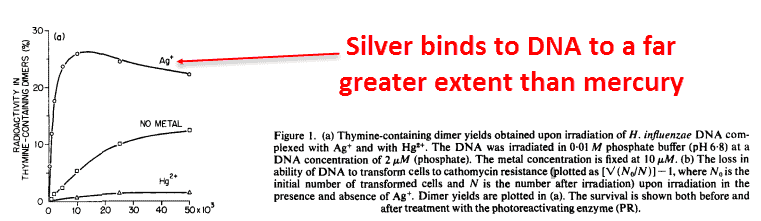
The DNA-binding properties of metal ions neatly correlate with their effectiveness against bacteria.
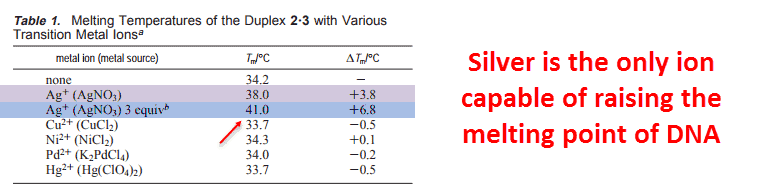
And silver is the only one that binds strongly enough raise the melting point of isolated DNA, keeping it in solid form longer with a link between the 2 strands — the semi-helices:
In all 3 review articles on the chemical mechanism of silver — those of Landsdown, Russell, and Thurman — thiol groups on proteins are usually given some consideration.
But this can’t be very important, since mercury and selenium bind more strongly to enzyme thiol groups than silver does…
…yet silver is more toxic to bacteria than both of these.
And it cannot explain the selectivity to bacteria — both humans and bacteria have thiol groups.
But can DNA binding explain the selectivity? Don’t humans and bacteria both have DNA?
Yes they do, but it’s different in humans. The cytosine in human DNA is mostly methylated, while in bacteria it is not.
And it’s been long-observed that silver binds to cytosine-guanine pairs the most.
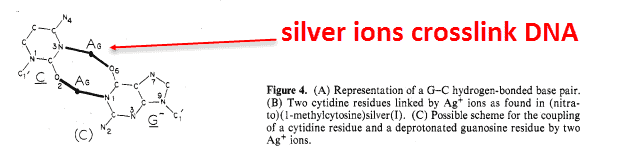
And just recently, researchers showed that silver binds less to the methylated form — the form found higher in humans:
These researchers were simply attempting to make a sensor: to use the different binding affinities between cytosine and methylcytosine to know what DNA type is present.
They found that silver bound to regular DNA, bacterial cytosine more.
‘…we show that in the presence of Ag⁺, duplex stability is most increased for the cytosine-cytosine pair, followed by the cytosine-methylcytosine pair…’ ―Wang
This could potentially explain silver’s selectivity, and its safety in humans. Methylated DNA protects mammals from silver ions.
Besides this, we have more direct proof that silver destroys bacteria by binding together its DNA — something that no other metal ion can do to any great extent.
In a meticulous experiment, Modak basically proved this.
He analyzed the cellular silver concentration, both in DNA and elsewhere, and compared this data to the growth curves.
He found that growth inhibition was perfectly correlated to the amount of silver bound to DNA, and growth resumed after it was diluted away.
And he found that the growth would sometimes increase when Ag⁺ was on the membrane — and not in the nucleus:
‘The percentage of the silver binding to the cell protein fraction remained constant or slightly increased during inhibition and growth.’ ―Modak
This is humorous because in all 3 review articles on the chemical mechanism of silver — those of Landsdown, Russell, and Thurman — its role on the cell membrane is also given consideration.
But this is most likely an artefact from electron microscopy — where the silver ions can give the false impression of the membrane ‘having separated.’
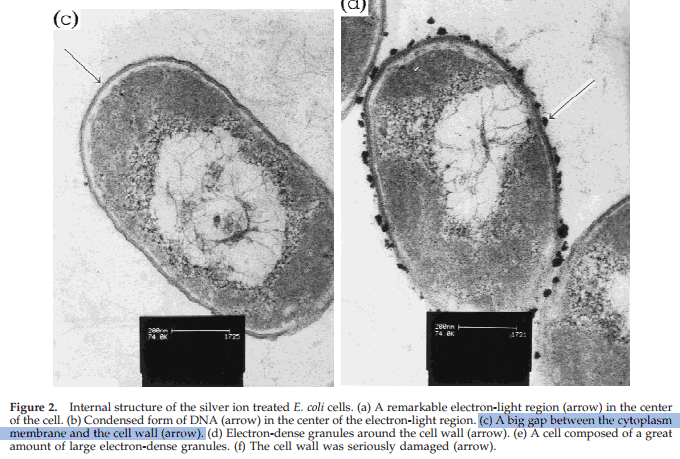
The scientist Harold Hillman often talks about how artifacts, like metal ions and formaldehyde, create imaginary structures.
This also shows how few of the articles many scientists cite have actually been read by them.
‘The data suggest that inhibition of bacterial growth results from interference of DNA function by binding of silver ions along the helical chain.’ ―Modak
Just as telling: Modak had shown a 10 times greater affinity for the DNA over the rest of the cell, including the membrane:

The methylated cytosine which apparently protects mammalian DNA from silver is not equally dispersed along the strand, yet is found in certain high-cytosine regions called CpG islands.
These areas are methylated the most: about 70% in mammals, yet only 5% in bacteria.
This is important, because methylcytosine in a high-density can promote the Z-DNA configuration — a radically different left-handed helix.
‘Methylation of cytosine in the C5 position is associated with a relative destabilization of the B-DNA structure and a stabilization through hydrophobic bonding of the Z-DNA structure.’ ‐Fuju
This could further explain why silver is safe for humans, yet deadly for bacteria, yeast, and certain parasites.
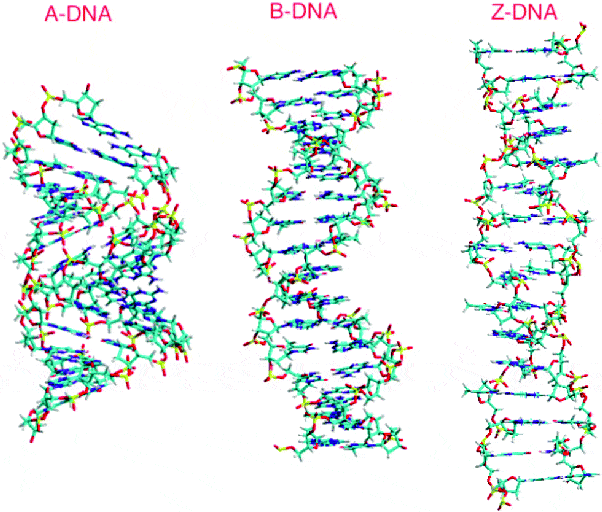
Despite this, most human DNA is modeled in B-configuration.
So it’s not the thiol groups, the cell membrane, nor anything else: silver is obviously working by tightly binding DNA together, thereby preventing its replication.
So colloidal silver really is a good antibiotic, both for its broad effectiveness and high selectivity.
The only ‘woo’ surrounding this topic is the dubious promotion of machines making ‘low particle size.’
As far as I can tell, all electrically-generated silver produces the same-sized ion (Ag⁺).
‘Following the empirical use of silver in classical antiquity, Ravelin in 1869 was the first to report on the very low concentrations at which silver and other metal derivatives could exert their antimicrobial effect. The word ‘oligodynamic’, literally ‘active with few’ (molecules, ions) was coined by von Naegeli in 1893 to denote this activity . . . von Naegeli found that a concentration of silver ions, derived from metallic silver, of 0.0000001 %, equivalent to 9.2 x 10⁻⁹ M, would kill the common freshwater Spirogyra. At a concentration of 0.00006%, equivalent to 5.5 x 10⁻⁶ M, the germination of Aspergillus niger spores was prevented.’ ―Russell
So if you’re looking for a highly effective antibiotic, it seems that you could do a lot worse than colloidal silver.
HOWEVER, all that may be fine, but remember that silver is a heavy metal. It accumulates in tissues. And it should be treated with GREAT respect.
—-Important Message for Men Who Want to Avoid Flus and Viruses—-
Believe it or not, flu shots are only 14% effective at preventing flu viruses…
…that’s why I’m using my own Flu and Virus protocol that is working for me in two ways:
- Helping my body naturally resist viruses on its own…
- and by counteracting the cytokines that are responsible for terrible symptoms and even death if I do get sick
I like to think of my Flu & Virus Protocol like a limiter on my car engine.
The limiter won’t let me rev the engine so high that it breaks the engine…
Well, same thing with my Protocol.
I believe it will rev up my immunity — but that it won’t let my immunity get so get out of hand that it kills me with the dreaded cytokine storm.
And right now, I’m letting other men try it for free — click here
———-
