
This Harvard doc’s trick can give men stronger rockiness

—-Important Message From Our Sponsor—-
Have you heard of the “Xersizer”?
It helps you give jaw-dropping performances to your wife, regardless of your age.
This Harvard trained doctor shocked the world with this discovery that helps men get the job done by improving penile blood flow.
There’s a bit of science involved…but this super easy trick turbo-charged my performance big time!
Here’s how to unleash better blood flow “down there” and perform at your best with her.
———-
What you need to know about chromium (is it good to take it, or not?)
The element chromium belongs within the small class of antidiabetic minerals, along with vanadium, magnesium, and zinc.
These elements are important enough that I’d recommend a trace mineral supplement that contains all four, but especially one including chromium.
What sets chromium apart is its incredible potency and safety.
A mere 200 micrograms per day has been found effective… And over 500 times that has actually been shown to be safe.
“These data demonstrate a lack of toxicity of trivalent chromium, at levels that are on a per kilogram basis, several thousand times the upper limit of the estimated safe and adequate daily dietary intake for humans.” (Anderson, 1997)
It should be mentioned that this observation applies only to trivalent chromium (Cr3+).
Hexavalent chromium (Cr6+) is less effective and potentially dangerous.
This isn’t likely to be a practical issue, however, as all current foods and supplements containing chromium only contain trivalent chromium.
Chromium is also rare in vegetables and grains grown in the US – and in animals that eat those plants.
This is especially true of the non-organic types because chromium isn’t usually added to fertilizer (de Souza Araújo, 2014).
For these reasons, chromium could be the most important mineral to supplement when consuming a normal U.S. diet.
The biological effects of magnesium and chromium are so profound that their combined deficiency (along with excessive omega-6 fatty acids) could be the prime cause of diabetes.
Another fact that makes chromium unique is that its ability to improve diabetes is its only known function.
All the other antidiabetic minerals are also known for other things, even vanadium.
So if any one element deserves to be called “the antidiabetic mineral,” it would probably be chromium.
Although vanadium got that title first (Lyonnet, 1899), chromium has been consistently shown more effective for over sixty years (Schwartz, 1959).
In fact, chromium is so effective that an organic form isolated from brewer’s yeast was originally known as the “glucose tolerance factor” (Anderson, 1977).
And after you see what chromium can do, and how it works, I think you’ll agree:

Large-scale clinical trials provide some of the best evidence for a treatment or supplement’s efficacy.
So it’s good to start with a meta-analysis that combines the results of many trials.
The reviewers of this particular meta-analysis analyzed 15 individual studies all using chromium picolinate (CrPic), the most bioavailable form commonly sold.
All studies under review had shown high efficacy with the exception of two, and both of those studies were fatally flawed.
“Thirteen of 15 clinical studies involving a total of 1,690 subjects (1,505 in CrPic group) reported significant improvement in at least one outcome of glycemic control.”
One of the studies showing a null result used a mere 200 μg (micrograms) of chromium picolinate per day.
Although this may initially sound like the normal dose, it is not.
The amount commonly used in clinical trials is actually 200 μg of chromium, as part of chromium picolinate – not “200 μg of chromium picolinate.”
200 μg of chromium picolinate equals just 24.86 μg of elemental chromium, while 200 μg of chromium (the “normal dose”) translates into 1,609 μg of chromium picolinate.

The very same type of error had actually occurred on a separate occasion.
And the authors were forced to admit that after being exposed in a letter to the journal that published the study (Komorowski, 2005).
The one other study showing a null result had used subjects who were all on insulin, which makes me suspect that the subjects were type 1 diabetics.
After knowing about chromium’s mechanism of action, there are reasons why it shouldn’t work in a small subset of extreme diabetics.
For example, it’s been shown that chromium supplementation increases pancreatic beta-cell mass by 41% (Hubner, 1988).
This is an overlooked finding that can partially explain its mechanism of action.
Type 1 diabetics have antibodies against beta-cells, meaning they’d be quickly destroyed by the immune system should new ones develop.
In other words, a chemical substance that partially works by increasing beta-cell mass wouldn’t necessarily work in people who lack beta-cells.
So after ignoring the minority of flawed studies, what remains is a praiseworthy demonstration of chromium’s efficacy.
Yet, on account of the wide range of doses and study periods between the individual trials, it’s somewhat untenable to statistically combine the results.
Nonetheless this had been done.
And the results of all 15 studies are shown averaged below (lower row.)
Not every study had measured every single parameter, but they all reported at least one:
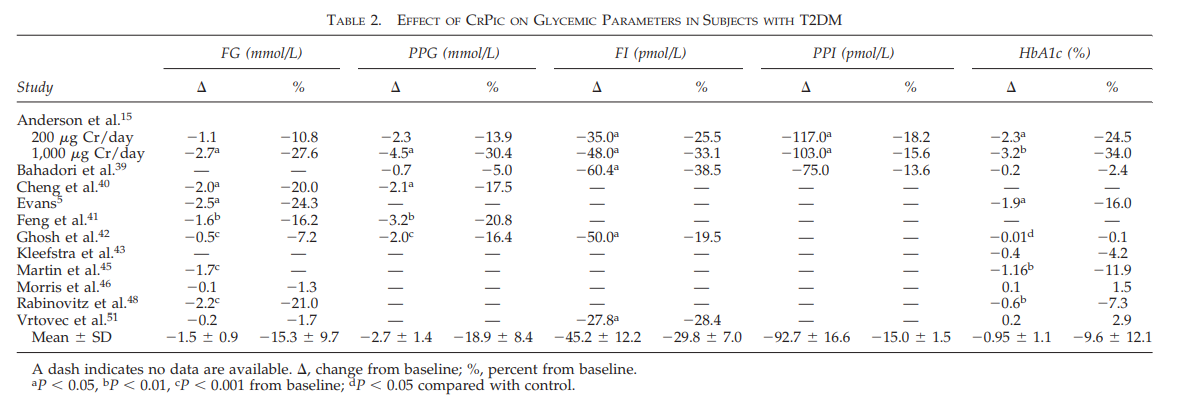
Five of the most common diabetes parameters are shown in the chart above:
- Fasting glucose (FG)
- Postprandial glucose (PPG)
- Fasting insulin (FI)
- Postprandial insulin (PPI)
- Glycated hemoglobin (HbA1c)
As you can see, chromium is more effective than just about anything in improving diabetes.
And, as with most effective antidiabetics, supplemental chromium has also been shown to translate into lower body weight (Martin, 2006).
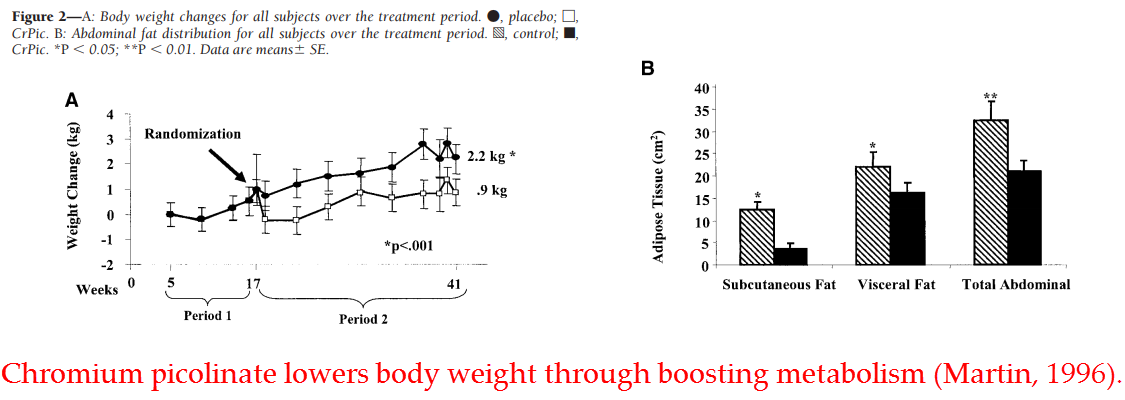
So chromium consistently improves glucose utilization in human subjects…
But the evidence doesn’t end there…
Diabetics have been shown to have lower levels of chromium in their hair and nails, on average. And chromium-deficient diabetes has been induced in rodents and humans.
There is also a dizzying array of in vitro evidence showing that chromium enhances glucose uptake – and only in the presence of insulin.
For these reasons, chromium is considered an essential mineral by some authorities and an insulin sensitizer by others.
So there’s certainly no lack of evidence proving that chromium is a powerful antidiabetic agent.
But how much chromium should we take? And in what form?
It’s been conclusively shown that chromium per se is the active agent (Mertz, 1960), with only the bound chemical species influencing its bioavailability.
In regards to the latter consideration, chromium picolinate (as mentioned above) has been shown to be the most bioavailable among nine chromium compounds tested (Anderson, 1996).
Although the nicotinate-glycinate chelate actually led to greater kidney concentrations of chromium, the picolinate complex better increased the concentrations in the liver.
Chromium in the liver is what matters most, because whatever is measured in the kidneys is soon excreted.
Moreover, the liver is considerably larger than the two kidneys combined – which translates into more chromium.
The liver also synthesizes a very specific protein that transports chromium directly to the insulin receptor.
And this fact helps to explain both its high potency and considerable safety.
Originally thought to have primarily a detoxification role, the “low-molecular-weight chromium-binding substance (LMWCr)” was discovered in the early 1980s after potassium dichromate injections were given to mice.
Potassium dichromate actually contains hexavalent chromium (Cr6+) – the toxic version – so it originally did make sense to view LMWCr as a detoxification protein.
(On the other hand, trivalent chromium (Cr3+) is so safe that using the term “detoxification” to describe its excretion would be inappropriate.)
Yamamoto discovered that these injections of potassium dichromate would induce the synthesis of an approximately 1,500 kilodalton (kDa) sized protein in the liver.
This protein specifically binds chromium, transports it to the plasma, and excretes it via the kidneys.
“It seems likely that the LMWCr may play an important role in the detoxification of excessive absorbed chromium and also in the availability of chromium as an essential element in the body.” (Yamamoto, 1981)
Although a peptide of this size wouldn’t always be considered as having “low molecular weight,” Yamamoto used the term only to differentiate it from the high-molecular-weight chromium-binding substance.
In fact, LMWCr is actually very similar to glucose tolerance factor (GTF) in composition…
Two cysteines, two aspartates, four glutamates, and two glycines are all that constitute it.
We now fortunately have a less lengthy name for this peptide than “low-molecular-weight chromium-binding substance” (LMWCr).
And it is also quite fitting – and easier to remember.
Twenty years ago, it was proposed that LMWCr should instead be called “chromodulin” – by analogy to calmodulin (calcium-modulated protein).
Both peptides bind exactly four of their respective atoms and exert regulatory functions (Vincent, 2000).
The name “chromodulin” has stuck, slowly superseding the term “low-molecular-weight chromium-binding substance” (LMWCr).
So now that you know about chromodulin, let’s have a look at what it can do:
Chromodulin has been shown to have greater affinity for chromium (Cr3+) than transferrin (Yamamoto, 1984), and hence is the body’s main chromium carrier protein.
So it was only a matter of time before somebody tested it on the insulin receptor.
After all, chromium’s antidiabetic action had been known for decades. And the insulin receptor is the body’s main antidiabetic target.
These scientists from the Deep South were not disappointed…
They discovered that chromodulin could potentiate insulin’s effect eightfold in nanomolar concentrations.
This effect was defined in the most rigorous way as protein tyrosine kinase activity.
(Tyrosine kinase is an enzyme.)
This is the manner in which insulin transmits a signal to the cell’s interior…
The insulin receptor is also a kinase enzyme which phosphorylates IRS-1 (insulin receptor substrate 1) upon activation.
This effect was not shared by any other element tested.
And this proves again why chromium is the most antidiabetic mineral.
Magnesium works only after insulin draws it into the cell. And vanadium acts solely by increasing thyroid hormone.
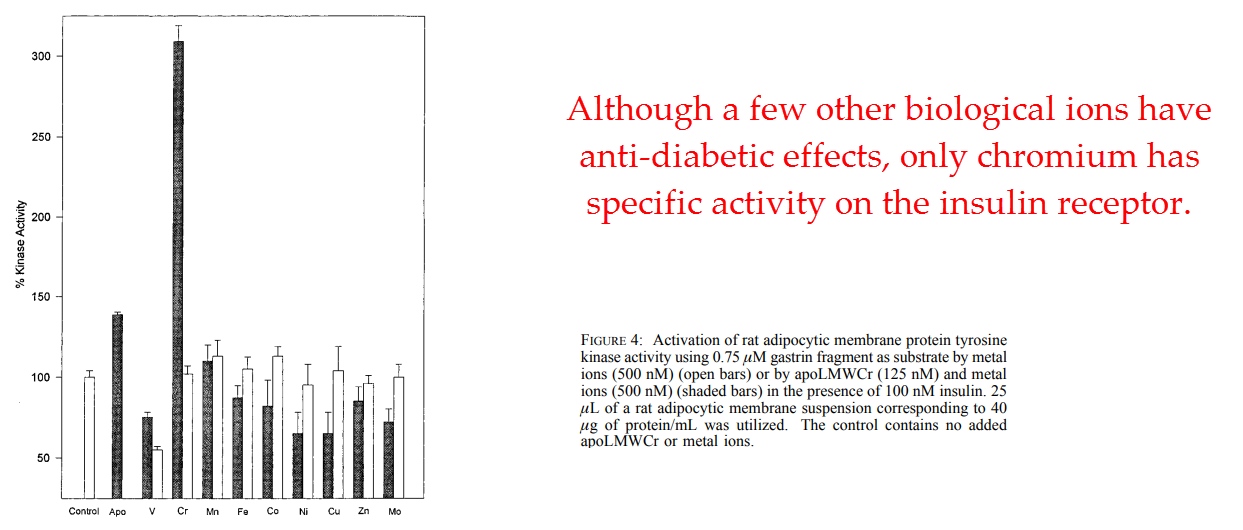
They also showed that this effect of chromodulin potentiating insulin’s effect through protein tyrosine kinase activity is strictly an insulin-mediated effect.
They convincingly proved that chromodulin’s effect is confined to the insulin receptor.
In addition, chromodulin was shown to bind the insulin receptor with a dissociation constant of 250 picomolar (pM), a value revealing the extraordinary affinity they have for each other.
Yet, before assuming that chromodulin acts like an insulin mimetic, check out their follow-up study:
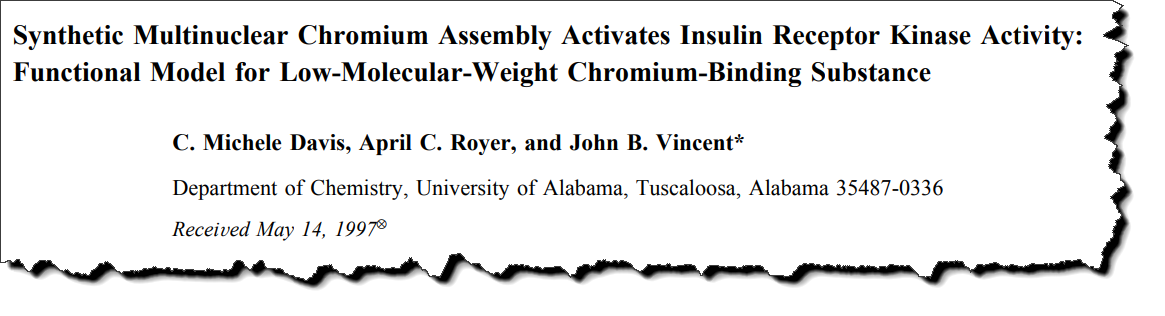
Using only half of the protein known as the “insulin receptor,” they were able to show that it binds the intracellular beta-chain and not the extracellular alpha-chain.
In other words, chromodulin does bind where insulin binds.
But instead it activates the enzyme portion – the tyrosine kinase.
The binding affinity towards the beta domain was measured at 133 pM.
This value is slightly greater than that towards the whole “insulin receptor.” (When it comes to dissociation constants, the lower value means greater affinity.)
Moreover, chromodulin was shown to enhance insulin-like growth factor (IGF-1) activity in the same manner as insulin.
IGF-1 is similar to insulin in more than one way, not least of which is its signal transduction mechanism.
Just as with insulin, the receptor for IGF-1 works through a tyrosine kinase enzyme.
Yet, despite its high affinity, they also showed that chromodulin isn’t entirely unique among chromium compounds in effect.
A crude chelate of chromium with the same amino acids and in the same ratio had activity almost identical to that of chromodulin:
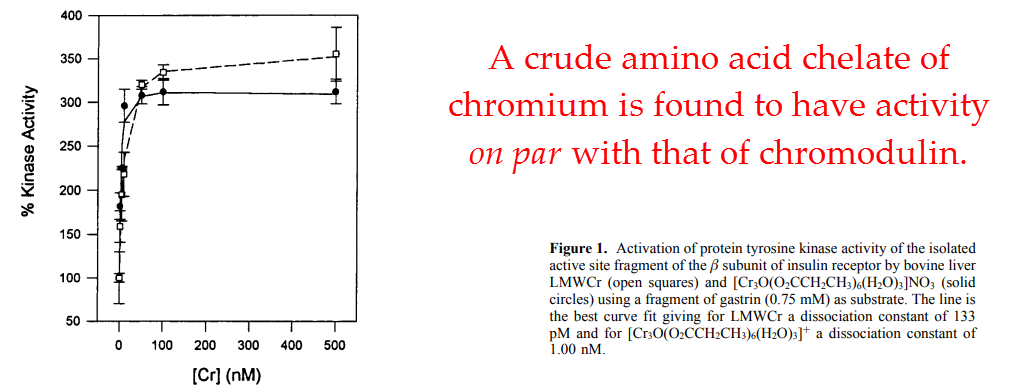
In line with this observation, they also found an inorganic chromium molecule with considerable activity.
When combined, these two studies by Davis and Vincent suggest that chromium potentiates insulin’s action by serving as cofactor for insulin receptor tyrosine kinase.
Other forms of chromium obviously work, especially after a long delay period.
Yet the neutral amino acid chelates work the best because they readily penetrate the cell membrane and have additional affinity for the insulin receptor beta-chain.
“The ability of LMWCr to stimulate insulin receptor tyrosine kinase activity is dependent on its chromium content.” (Davis, 1997)
Considering the scarcity of dietary chromium, it would make sense that the only enzyme that requires it would evolve with the ability to bind its carrier protein.
In this way, the insulin receptor could be synthesized peripherally and then given its cofactor whenever chromium is consumed.
This would also answer the million-dollar question…
Why chromium? Why would this mineral, of all things, be chosen by the body to behave like insulin?
Chromium is not particularly associated with high-sugar foods. And effective amounts are rather difficult to come by.
Is diabetes the universe’s way of punishing people who don’t eat canned apples, a food that contains a particularly high amount of chromium?
Of course not!
Like all minerals, chromium was likely “chosen” because it most efficiently catalyzes the tyrosine phosphotransferase activity needed to transmit insulin’s signal inside the cell.
All atoms have different redox potentials, electronic configurations, and molecular affinities.
So there is not just one that can efficiently catalyze all biochemical reactions.
“The glucose tolerance factor is a dietary agent required in rats for maintenance of normal removal rates of glucose from the blood. Recently, we have identified trivalent chromium as the active ingredient.” (Mertz, 1960)
And since chromium is considered essential by most authorities, there’s actually no reason not to take it.
Chromium deficiency has actually been shown to cause a disease, and one disease in particular – diabetes.

It seems that mild chromium deficiency is somewhat common.
But extreme deficiency is relatively rare because of the ubiquitous presence of stainless steel in cookware and canned foods.
We also inhale chromium to a considerable degree in large industrial cities.
So a near-total deficiency has only been reported in people being fed 100% refined foods in controlled environments.
Although chromium-deficient diabetes had been induced in rats before now (Schroeder, 1966), this study was the first report of the condition in a human subject.
The authors detail the case of a woman who progressively lost weight during parenteral feeding (injection or infusion feeding).
Eventually she was found to have a glucose clearing rate of only 74% of the minimum that’s considered normal.
“These events were associated with low levels of chromium in blood and hair, with abnormal intravenous glucose tolerance and with an increased caloric requirement for maintenance of weight.” (Jeejeebhoy, 1977)
Her respiratory quotient was also .66, suggesting that her body was using fat for fuel despite a daily infusion of insulin and glucose.
Most patients in this condition would be prescribed pharmaceutical drugs and perpetually milked for cash…
But, since this one was on a synthetic diet given by a concerned doctor, a nutritional cause was looked into.
They checked for chromium concentrations, no doubt because of chromium’s reputation for reversing diabetes.
And they found the patient’s chromium to be extremely low:
Plasma chromium levels were only 11% of the lowest level considered normal, and hair concentrations were 33% of the lowest level considered normal.
For these reasons, they put her on 250 μg (micrograms) of chromium per day…
And a resolution of her diabetic state occurred in just a few days.
After two weeks her respiratory quotient had nearly doubled to 1.35, well within the normal range, and the patient no longer needed insulin.
“All these features were corrected or restored to their previous equilibrium by chromium supplementation.” (Jeejeebhoy, 1977)
So chromium is certainly the antidiabetic mineral – if that can be said of any mineral.
Chromium had been consistently shown to be uniquely effective in reversing diabetes since 1959.
The only situations where chromium doesn’t work to reverse diabetes:
(1) Subjects are already chromium sufficient
(2) Too low of a dose is used
(3) It’s given to people lacking the ability to synthesize chromodulin
(4) It’s given to people with insulin receptor autoantibodies
Chromium picolinate is a safe, readily available, and highly bioavailable form.
Beneficial effects occur with only 1.61 milligrams per day (equal to 200 micrograms (μg) of chromium per day).
200 μg is the official upper intake level…
But chromium picolinate is safe enough that I don’t feel irresponsible in stating that you could triple that value…
Especially considering that 1,000 μg of chromium has been used many times in clinical trials without untoward effects (Broadhurst, 2006).
“The nutrient Cr has one of the largest safety factors of all nutrients and there have been no confirmed negative effects of supplemental Cr at intakes of 1,000 μg per day or more…”
“The reference dose defined as ‘an estimate…of a daily exposure to the human population, including sensitive subgroups, that is likely to be without an appreciable risk of deleterious effects over a lifetime’ is 70,000 μg per day. The maintenance dose employed in this study was 200 μg per day and the highest level employed was 600 μg per day for 2 weeks.” (Ravina, 1999)
—-Important Message—-
Touch her and make her come with this weird electrifying method

It sounds strange, but this weird electrifying method shocks a girl’s pleasure centers in a way she’s never experienced before…
Just a touch of your finger will have her eyes rolling into the back of her head…
…and she will shudder with the waves of pleasure crashing throughout her body…
And when she opens her eyes, you’re the only man she sees…
———-
