
Aluminum is hiding in sneaky places, even our water!
—-Important Message From Our Sponsor—-
Push this vulva spot and watch what happens — “I could feel my juices running down my legs”

You’ve got to hear this fascinating story of Joyce Anderson… and how her husband did something radically different that rocked her body in ways she never imagined…
You see, as his fingers entered her and touched her in a new, exciting way…
She saw ribbons of light flash behind her closed eyelids and she could feel her chest heave…
Her thighs began to quiver, and her back arched as a shockwave of ecstasy surged through her whole body…
This new sensation wasn’t like any kind of pleasure she’d felt before and all Joyce knew in that moment was she didn’t want him to stop.
She could feel her stomach contract and her breathing grow shallow while her deep moans of pleasure rattled the air…
And as the intensity reached its peak… a series of contractions seized her in between her thighs and then…
She released… a stream of her juices gushing out like a high-powered sprinkler…
The only thing that kept her from losing consciousness was another wave of prickling pleasure seizing control of her body…
Until she collapsed like jello, a wet puddle of her juices soaking their bed sheets…
Here’s how to do this to your wife or girlfriend in 38 seconds flat.
———-
How Aluminum causes Alzheimer’s — and how to stop it
Aluminum is a neurotoxin routinely added to food, and is correlated with Alzheimer’s disease so consistently as to be considered its prime cause.
This form of dementia has been correlated with aluminum in drinking water, a neurotoxin added intentionally at many water treatment plants as a flocculant.
Yet the same epidemiological studies show an inverse relationship between the dementia and the water’s silica content.
These results have spawned a good deal of speculation, from the “reduced uptake of aluminosilicates,” to just “coincidental properties between city and well water.”
That is, water that demands treatment with flocculants is often recycled and expected to be low in silicates for that reason.
Conversely, deep well water is high in silicates yet is clear, negating the very need for added flocculants.
These two strong trends have created a schism from the start, with some scientists studying aluminum’s toxicity while others focused more on silicic antidote.
Yet regardless of the relative contributions of each, lowering the aluminum∶silicon ratio in the diet would be unanimously approved.
And there is some confusion over “silica.” This is the primary chemical constituent of sand and glass, and as we all know this is insoluble in water.
Yet the form it takes in water, silicic acid, is quite slightly different and IS soluble. This small molecule has a peculiar affinity for aluminum and nothing else.
Hydrated silica is safe, soluble, and is definitely not the tiny crystals some people might suppose it to be.
It is common to simply use the elemental name for minerals in the field of nutrition, yet the forms they take in water and within the body are often more complex.
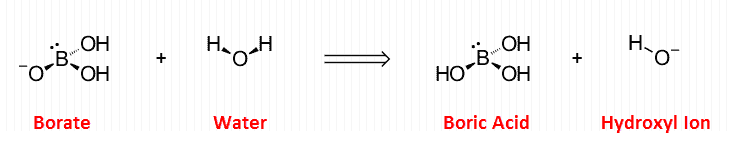
For example: “Boron” is useful for some forms of arthritis, but the specific form it takes within the body alternates between the boric acid ⇌ boric acid equilibrium pair.
When nutritionists speak of “boron,” they are probably referring to the hydrated form.
Because boron and aluminum are found in the same group on the periodic table, it’s intuitive that they should antagonize each other in plants (Jiang, 2009).
Boron has no known essential role in mammals, yet it does have beneficial effects.
Although boron-aluminum antagonism studies on mammals are lacking, borates are often worth taking anyhow for a different reason.
Borates have antiarthritic properties, perhaps through destroying yeast and fungi in the body.
They also have very low toxicity in the milligram range where they’ve been shown effective (Newnham, 1994), and not even massive accident-sized doses do any harm (Litovitz, 1987).
‘In conclusion, the majority of acute boric acid ingestions produce no toxicity. According to our data, boric acid ingestions produce minimal toxicity at serum boric acid levels of 340 μg/mL or less. A review of previously reported cases would indicate that much higher blood levels are well tolerated. Hemodialysis shortened the half-life of boric acid. However, considering the apparent lack of toxicity of acute ingestions, insufficient data are available to determine its value as a treatment modality aimed at preventing the subsequent development of serious clinical toxicity.’ ―Litovitz
Selenium is a bit more diverse and can assume multiple forms in solution, such as: hydrogen selenide (HSe−), selenate (SeO42−), and dimethyl selenide (Se(CH3)2).
Yet regardless of the precise species under discussion, the term “selenium” is still imprecisely used to describe it.
This practice has been taken to the extreme in the case of L-selenomethionine, a selenoamino acid sometimes referred-to as “selenium” by medical doctors (Lippman, 2009).
Selenomethionine has anticancer properties quite distinct from any other selenium species, making it an injustice to call it simply “selenium” (and also misleading.)
Selenomethionine inhibits prostate cancer through inhibiting polyamine synthesis by mimicking methionine, and also concentrates in the prostate due to that organ’s natural affinity for this amino acid.
Silicon is no exception, an element with multiple hydrated forms yet conceptualized as though it were monoatomic.
This element, after reacting with water, can also form polysilicates at high concentrations.
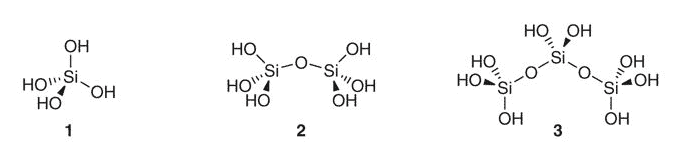
Yet at low bodily concentrations and physiological pH, you’d primarily only expect: silicic acid (1), its dimer (2), and the silicic anion (H3SiO4−).
These silicon species don’t interact with much of anything in biological systems besides aluminum and also have a good safety profile.
The average dietary intake has been estimated at 30 milligrams of silicon per day (Birchall, 1995), mostly found as insoluble silicon dioxide (SiO2) in plants.
Yet there is reason to suppose that quite a bit of this ingested silica (SiO2) is hydrated into silicic acid (H4SiO4) in the intestines (duodenum), thereafter being absorbed into the bloodstream.
‘The total human ingestion has been estimated to be 30 mg Si per day with 60% of this from cereals and 20% from water and drinks, and with the latter providing silicon in its most bioavailable form as silicic acid.’ ―Bellia
The low toxicity of the most bioavailable form, silicic acid, is a testament to its safety.
With an LD50 of 4.5 grams per kilogram, it would take 315 grams consumed at one time to kill ½ the people in my weight class.
Excess silicic acid is quickly excreted, and beer has the highest amounts (Bellia, 1996).
So silica and silicic acid are quite safe and they are, in fact, normal dietary components.
Even though silicon has no known role in biology, in any form, its deficiency has been viewed as pathological in classic animal feeding studies.
Yet decades of newer evidence suggest its once elusive function derives fundamentally from its antagonism of aluminum, and nothing else.
It is now thought to increase the activity of collagen forming enzymes by preventing iron and copper from being displaced by aluminum.
This mechanism seems plausible, and could be the reason behind aluminum’s negative effects on bone growth.
‘The biochemical mechanisms underlying the effects of silicon deficiency have until recently been obscure, with no evidence for any organic binding of silicon. Recent studies have shown that silicic acid, Si(OH)4, the form in which silicon exists in physiology, interacts with aqueous aluminium species to form hydroxyaluminosilicates […]’ ―Birchall
It’s been long-known that silicic acid inhibits the absorption of aluminum into the body, yet the idea that it could affect its elimination had remained tentative until just recently.
Now we have proof that silicic acid can do more than just inhibit Al3+ uptake.
It can also increase its rate of elimination:

This study had given memory-impaired patients Volvic® mineral water and were instructed to drink all 1.5 liters per day.
Most subjects were fully compliant, yet a few only drank only one-half that amount.
Nonetheless, they had gathered convincing data that body stores were declining.
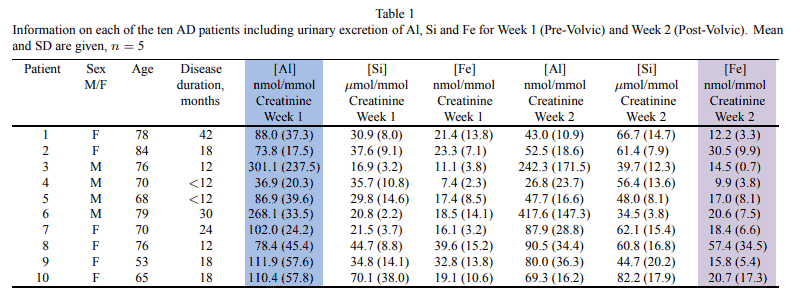
This is just what you’d expect with a declining aluminum body burden, yet the urine samples were collected at the worst time imaginable.
Most of the silica in this study was consumed during the day, slowly and spaced-out, while detailed kinetic experiments show that the majority of aluminum is excreted immediately after consumption.
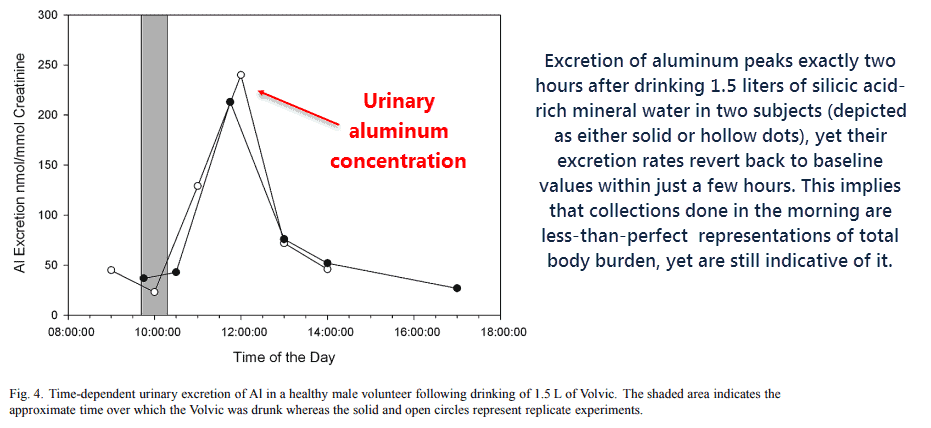
Other studies including the same design report similar results.
Yet the excretion data measured directly after consumption, when actually done, consistently shows a significant spike in aluminum output within the first few hours.
So you can bet the farm that aluminum is actually being eliminated, despite morning samples being somewhat variable.
The increases in the apparent “body burden” in two subjects could’ve been the result of a redistribution of internal body stores, perhaps out-of the tissues or nerves.
This elimination pattern was noted previously in one subject with multiple sclerosis.
And there’s also indication that the aluminum found in neurofibrillary tangles, the most resilient form, can actually be removed using chelating agents.
‘Incubating the aluminum-treated tau samples with deferoxamine reversed the effect of aluminum on tau mobility, causing tau to migrate similar to the untreated control sample.’ ―Scott
This means that Alzheimer’s could be reversible…though I assume anyone reading this still has time to prevent it.
Dimers of silicic acid have affinities towards aluminum on par with deferoxamine, a synthetic chelating agent, implying that it could be just as powerful.
Yet unlike deferoxamine, silicic acid leaves other metals alone.
And there is even one study showing that silicic acid can revert misfolded brain proteins back to their normal α-helical state:

This study had used brain proteins normally found aggregated in Alzheimer’s disease.
The researchers first confirmed that aluminum could aggregate the protein, as had been shown previously, by crosslinking it through its phosphate groups.
Aluminum has a natural affinity for phosphate, making it intuitive why neurofibrillary tangles tend to include highly phosphorylated proteins.
They confirmed these changes through circular dichroism, an analytical technique that discriminates between angles of plane-polarized light.
‘Previous studies had shown a conformational transition from α-helix and random to β-pleated sheet upon addition of Al3+ to both phosphorylated and unphosphorylated peptides. If sufficient quantities of Al3+ are added, the peptide precipitates from solution.’ ―Fasman
Certain molecules in solution will rotate a plane of polarized light, giving researchers insight as to its molecular geometry. This technique is very reliable.
They found that aluminum transforms the proteins’ normal shape into a β-sheet configuration, and also that sodium silicate could reverse it.
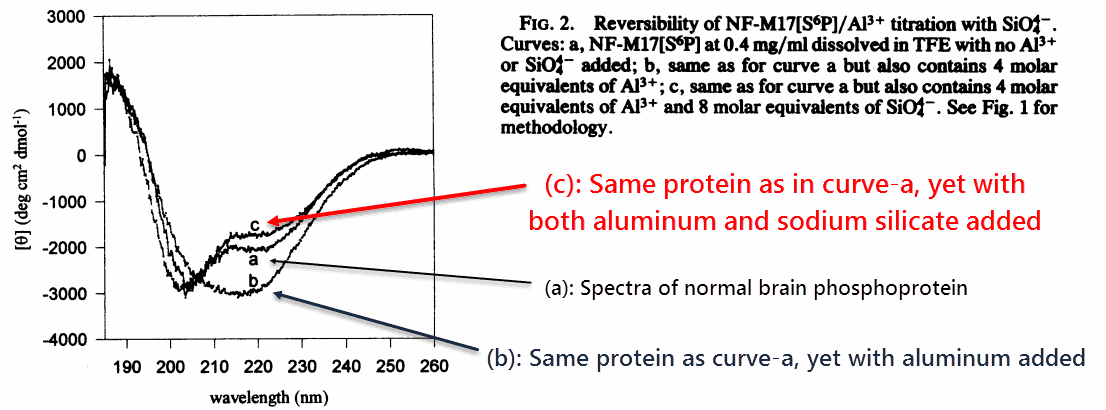
Sodium silicate didn’t merely “prevent” it, but actually reversed it. The silicates were added AFTER the aluminum had crosslinked the protein.
‘At equal concentrations of Na4SiO4 and Al3+ the peptide CD spectrum was beginning to resemble the original CD spectrum.’ ―Fasman
So there is every indication that silicic acid — “hydrated silica” — can eliminate aluminum from the body, yet evidence also suggests it could help dissolve aluminum-crosslinked brain tangles.
Although concentrations in the plasma don’t usually achieve the level to compete with citric acid and transferrin, the two primary aluminum-binding molecules, it doesn’t matter because this is not how it works.
Silicic acid is very rapidly absorbed and eliminated, the reason why kidney concentrations are the highest in the body.
In the kidneys, silicic acid has been shown to enhance aluminum excretion by reducing its reuptake.
Although the concentrations of silicic acid in the cerebrospinal fluid is always lower than in the kidneys, they are higher than in the plasma.
The relative absence of citrate in the brain could make silicic acid a force to be reckoned with, our primary “brain chelator.”
‘In the cerebrospinal fluid with little citrate and no transferrin, Al3+ is complexed weakly by other ligands, and the tendency toward aluminosilicate formation becomes much greater than in the plasma.’ ―Martin
Citric acid also enhances aluminum excretion yet also increases its absorption.
Aluminum complexed with citrate is a neutral species, and thus has enhanced penetration through all membranes.
Even though most of the foods that are high in aluminum sound terrible with orange juice, such as frozen pizza and cupcakes, I think this is a caveat still worth mentioning.
Orange juice should only be consumed with low-aluminum foods.
Silicic acid, on the other hand, enhances the elimination of aluminum AND reduces its absorption.
For this reason alone, but also its perfect safety profile, silicates should be the first line of defense against aluminum neurotoxicity.
—-Important Message—-
Food Warning to Men: I found this Alzheimer’s causing additive in virtually all foods

I’m blowing the lid of this well kept secret…
…how virtually every food is intentionally and knowingly contaminated with a toxic chemical that radically increases cancer…heart disease…even Alzheimer’s…
You won’t find this ingredient listed on the label unless you know where to look and how to read the label…
…it’s the most dangerous additive and you should avoid ANY food that has it…
I made this video to show you how to find the additive on the label, and what foods to avoid…
———-

Davenward, Samantha. "Silicon-rich mineral water as a non-invasive test of the ‘aluminum hypothesis’ in Alzheimer's disease." Journal of Alzheimer's Disease (2013) https://pdfs.semanticscholar.org/ed93/2fec74fa7648a834c15ab82f4d81ea3cf366.pdf
Swaddle, Thomas. "Silicate complexes of aluminum (III) in aqueous systems." Coordination Chemistry Reviews (2001) http://sciencedirect.com/science/article/pii/S0010854501003629
Fasman, Gerald. "The solubilization of model Alzheimer tangles: reversing the β-sheet conformation induced by aluminum with silicates." Proceedings of the National Academy of Sciences (1994) http://www.pnas.org/content/pnas/91/23/11232.full.pdf
Exley, Christopher. "Non-invasive therapy to reduce the body burden of aluminium in Alzheimer's disease." Journal of Alzheimer's Disease (2006) https://www.researchgate.net/profile/Stanislav_Strekopytov/publication/6806911_Non-invasive_therapy_to_reduce_the_body_burden_of_aluminum_in_Alzheimer's_disease/links/09e4150c1c238a2aad000000.pdf
