
Feminists hate men talking about this…
—-Important Message From Our Sponsor—-
An exclusive private gentleman’s club invites your participation — here’s why you want to jump at the chance
For too many years now, men have been made to look like idiotic, bumbling fools on television, at work, even in their very own home.
While secretly women disrespect and have disdain for the p**sy boys.
Well I have a solution… there is actually a private, exclusive club for the man who wants a woman who respects & cherishes him…even making sure that all of his “bedroom needs” are met…
…but you must dare to be the boss in bed.
This is a real gentleman’s club, like they have in England.

As you can imagine, membership to such an exclusive club is NOT cheap. Far from it.
However, the founder of the club has made the decision to allow 100 good, honest men who want to be active participants & follow the simple club rules…
…to have a FREE membership.
No charge today, no charge going forward. A FREE membership for life.
———-
How to speed up your gut, increasing vitality and potency…
A slow gut is probably the hallmark of a man with “rockiness” problems, a man who isn’t at his best.
And now it turns out that methane is becoming known as a prime cause of constipation (IBS-C).
The recognition of methane’s correlation with IBS-C has initiated a series of corroborative research.
And the results have implicated just one microbial species in producing the majority of IBS-C cases in humans (Kim, 2012).
Two separate studies have shown that the infusion of methane directly induces constipation (Pimentel, 2005; Jahng, 2012).
This confirms that methane is a cause of constipation and not simply an effect.
“In this model, small intestinal infusion of methane produced a slowing of transit in all dogs by an average of 59%.” (Pimentel, 2005)
These findings have led physicians and supplement producers to look for methods of decreasing methane production in humans.
Before we go on, you need to know about this organism:
- Methanobrevibacter smithii is a methanogen… It recycles hydrogen (by combining it with carbon dioxide), resulting in methane.
Dr. Mark Pimentel has proposed the use of statins to cripple M. smithii through the inhibition of HMG–CoA.
HMG–CoA is the rate-controlling enzyme of the metabolic pathway that produces cholesterol (among other things).
His justification for proposing this method is that is that M. smithii is an archaea and not a bacterium:
Archaea have cell membranes composed of isoprenoids rather than fatty acids, and are thus highly reliant upon the enzymes that synthesize them.
In fact, statins are one of the few drug classes that specifically inhibit archaea – common antibiotics are largely ineffective.
Another method to lower methane, albeit less specific, is the use of oxygen‑releasing agents.
M. smithii is an obligate anaerobe (organisms that can only live in environments without oxygen).
So M. smithii is highly sensitive to oxygen, even the small amount contained within normal air (about 21%).
Dr. Kenneth Brown has pioneered the use of tannins to lower methane.
Maybe he thought of this after coming across older literature about ruminants (cows, goats, sheep, deer, etc.)
Methane is a greenhouse gas and also reduces feed conversion (or efficiency).
For this reason, a good deal of research has been directed towards reducing methane.
Tannins are actually the most studied methane‑reducing agents, though the effect is somewhat modest and variable by type.
Tannins are generally divided into two main classes, hydrolyzable and condensed:
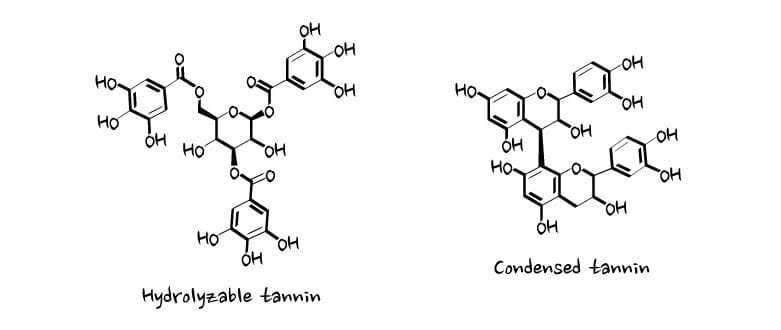
Both types are used to tan leather because they have the ability to bind proteins, but only hydrolyzable tannins are used to make ink.
This is because only hydrolyzable tannins effectively bind metal ions such as the ferrous iron contained in iron-gall ink.
This metal‑binding property is important, and is likely what underlies the ability of tannins to inhibit methane production.
M. smithii depends on nickel ions (Ni2+) to produce methane.
This is because nickel occupies the center of heme structures called cofactor F430, which is central to the process of the formation of methane.
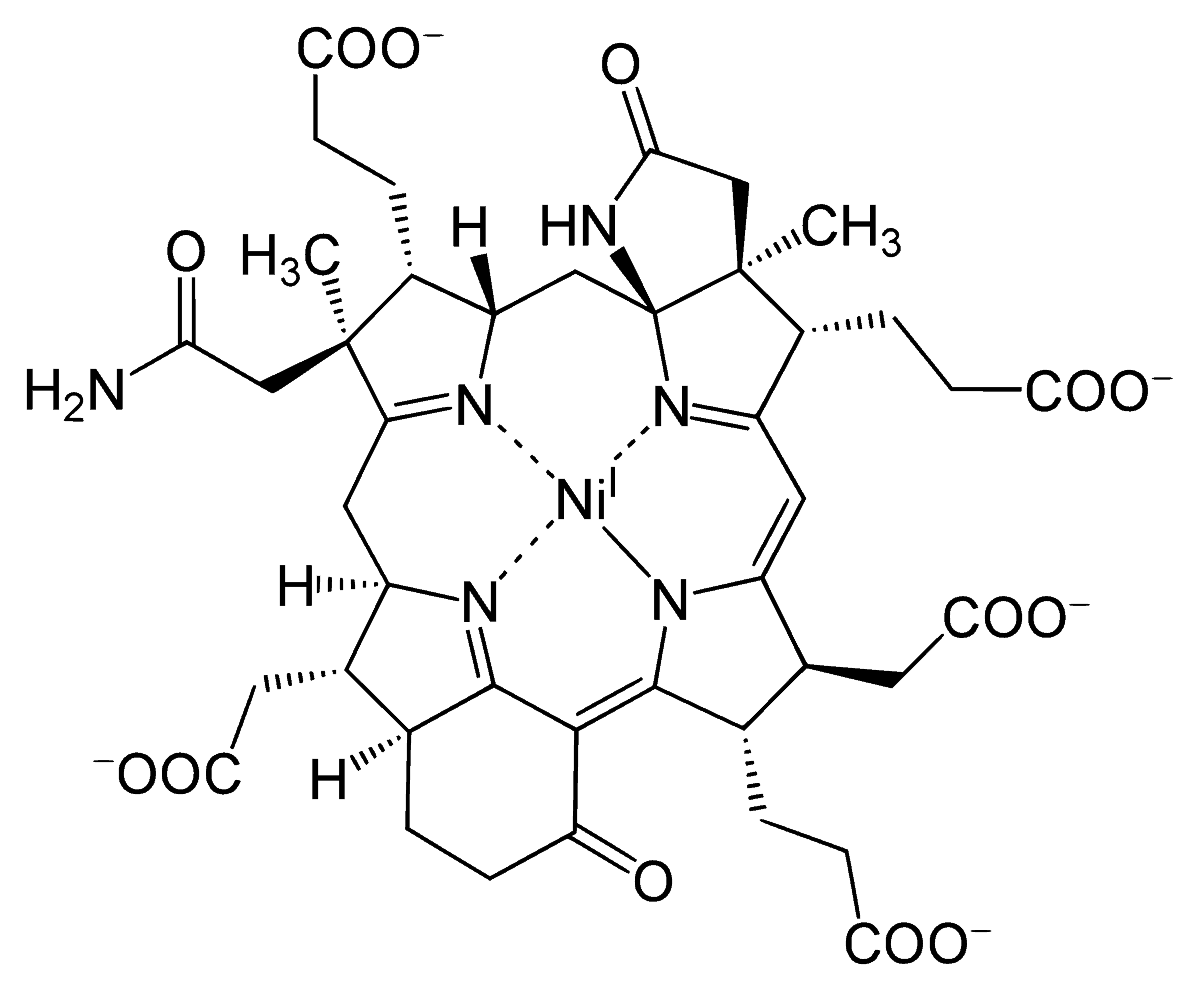
The central nickel atom is needed to adsorb the hydrogen (H2) that is later added to methyl groups of coenzyme M, thus releasing methane in the process.
Ni–H2 + 2·CoM–CH3 ⟶ CH4 + CoM–CoM
Nickel is also used to adsorb and hold hydrogen during the process of hydrogenation, usually on a high‑surface area substrate called Raney catalyst.
Only platinum and palladium share this unique property of nickel, but they are relatively rare and expensive.
Nickel seems to have an inherent affinity for hydrogen, with nickel hydrides actually being stable compounds.
So whether it’s bacterial or industrial, nickel is indispensable to the process of methane generation – by adsorbing hydrogen.
Yet, because hydrogen is just as important as nickel for producing methane, decreasing hydrogen-producing bacteria is also somewhat effective.
This fact introduces uncertainty into many methane‑lowering studies because you don’t know exactly what’s being inhibited or where.
“All methanogenic bacteria investigated were found to contain factor F430… It is therefore very probable that all species of methanogenic bacteria contain this nickel tetrapyrrole.”
More specificity in inhibiting methane is preferable, going beyond killing hydrogenic bacteria and binding all proteins.
Condensed tannins (such as those from quebracho) should probably be avoided in such studies for that reason.
Condensed tannins irreversibly bind proteins and inhibit their digestion. But hydrolyzable tannins do so only transiently.
Dividing the categories further, the subtypes of the hydrolyzable class of tannins are predominantly ellagitannins and gallotannins:
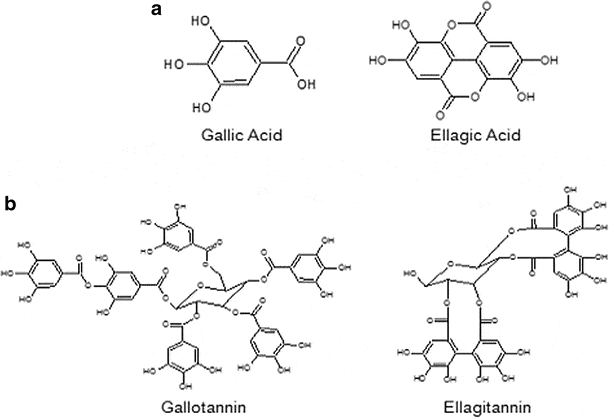
And of these two subtypes, ellagic acid has been found to have the best nickel‑chelation properties – while gallotannin associates better with proteins.
Because nickel restriction specifically impairs methanogens, ellagic acid seems the best tannin to take.
It has been shown that the metal-ellagate complex is insoluble through centrifugation – meaning that it won’t be absorbed into the body or used by bacteria.

Here are some studies that best exemplify this idea:
Coincidentally, this classic study used M. smithii, the same species shown decades later to account for essentially all methane production in humans.
They also tested the nickel requirement of other methanogens, and even a few species of non‑methanogens such as Acetobacterium woodii, Clostridium thermoaceticum, and Escherichia coli.
They found that only methanogens contain cofactor F430 and require nickel.
They also found that nothing else could substitute for nickel.
“In the absence of added nickel, M. smithii grew slowly and to a cell concentration of only 0.3 g/liter. When 1 μM nickel chloride was added to the medium, both the growth rate and the final cell concentration drastically increased. No other transition metal could substitute for nickel in this respect.” (Diekert, 1981)
So it seems that nickel is indispensable for methanogens – and only methanogens.
Although other bacteria use nickel for a limited number of enzymes, nowhere else does it occupy such a prime role as in methanogens.
In fact, methanogens produce the very energy they require for survival by converting hydrogen into methane gas.
Without nickel, methane‑producing organisms can’t even produce their eponymous gas.

These results were subsequently confirmed through methane production (Murray, 1981; Speece, 1982), rather than growth rates.
It’s actually profitable to produce methane for use as a fuel, and that’s why some are interested in how to increase it.
Nickel was found to be as specific for generating methane as for the bacteria that produce methane, which is to be expected from their biochemistry and enzymology.
Nothing can substitute for nickel for making methane – other than perhaps platinum or palladium, and certainly not cobalt or molybdenum (Murray, 1981):

Nickel increases methane production and the converse is also true…
Restricting nickel availability via chelating agents has been shown to greatly reduce methane emission.
This effect was demonstrated in South Korea in 2012:
This study used ethylenediaminetetraacetic acid (EDTA), a chemical that is used for many medical and industrial purposes.
They took mud from rice fields and measured initial methane emissions from the mud.
Every week of the study after that, they added EDTA at three concentration levels: 30, 60, and 150 parts per million.
They also determined the concentrations of cofactor F430 by chromatography and its gene counts via PCR.
All parameters tested – methane production, nickel enzymes, and methyl coenzyme M reductase A (MCR-A) gene expression – went down with increasing EDTA.
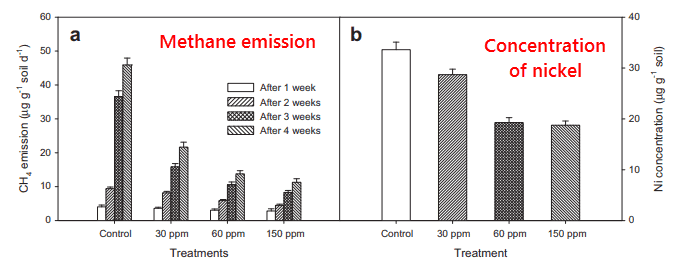
This effect wore off after a few weeks as the EDTA was metabolized.
But a steady supplementation of a nickel chelator should keep methane production low.
The fact that nickel chelators also bind iron can be seen as a benefit in light of iron’s effect on lipid peroxidation.
“Since the concentrations of heavy metals except that of nickel were not proportional to the rate of methane emission, it could be concluded that EDTA possibly most effectively limited nickel bioavailability to methanogens. And that in turn suppressed the activity of methyl coenzyme M (MCR) enzyme during methanogenesis.” (Pramanik, 2013)
And nothing seems more suited for binding nickel than ellagic acid, a dietary compound safely consumed by humans for millennia.
Although ellagic acid also binds cupric (copper), ferrous (iron), and zinc, there is some indication that it binds nickel most specifically.
This could be what underlies the historical use of ellagic acid in reducing methane emissions in ruminants.
Although a handful of studies show ellagic acid to effectively chelate ferrous, zinc, and cupric, this one is unique in studying its effect on the nickel ion.
They used tannins derived from oakwood, a source comprising predominantly ellagic acid and ellagitannins.
They confirmed chelation through high‑pressure liquid chromatography and mass spectrometry, a completely unambiguous way to demonstrate tannin–ion complexation.
They confirmed the substantial affinity that ellagic acid has for metal ions, and also implied that it binds the most strongly to nickel.
“…ion intensity ratios for other metal ions…also increased with total metal concentration. The increase was most pronounced for nickel which, like copper, forms stable complexes with many organic ligands.” (Ross, 1999)
Ellagic acid has also been successfully employed to chelate nickel during production of nickel oxide nanocrystals for solar cells (Yuvakkumar, 2014).
And it has been found more effective than the same dose of EDTA in preventing nickel poisoning in rats (Ahmed, 1999).
Nickel chelation could be what underlies the efficacy of tannins in inhibiting methane production.
Since nickel is a characteristic requirement of methanogens – not iron, copper, or protein – it stands to reason that ellagitannins should be more effective than gallotannins in lowering methane production.
And they should also be more effective than condensed tannins, which are perhaps only useful for binding proteins and making leather…
This study compared the effects of hydrolyzed and condensed tannins on in vitro methane production. (in vitro = in glass in the lab)
They collected fluid from cows’ rumens (cow’s first stomach).
And they mixed that with wheat bran, barley, soya, and tannins extracted from one of four sources: chestnut, sumac, mimosa, and quebracho.
In addition to measuring methane, they determined short‑chained saturated fatty acids, products of fiber fermentation.
In this way, they could compare the effects of lowered methane versus that of reduced feed conversion.
They also estimated cell populations of methanogens, fungi, and two species of bacteria via PCR.
Both tannin types inhibited methane.
But the hydrolyzable ones did so more, while having less effect on fatty acid synthesis.
“It is concluded that hydrolysable tannins had a greater effect in reducing methane emission with less adverse effect on digestibility than those of condensed tannins.” (Jayanegara, 2015)
The two types showed different dose inhibition curves, with hydrolyzable tannins inhibiting methane quadratically and condensed tannins doing so linearly.
This data can be inferred to represent two different modes of action.
From these foregoing considerations and the microbial cell counts, they concluded that hydrolyzable tannins were both more effective and more specific towards methanogens than condensed tannins.
The two hydrolyzable tannin extracts used, chestnut and sumac, were sourced from plants rich in ellagic acid.
Chestnuts are also used to make iron‑based ink, perhaps on account of the metal‑binding properties of their ellagitannins.
“The condensed tannins appear to decrease methane more through a reduction in fiber digestion, while hydrolysable tannins act more through inhibition of the growth and/or activity of methanogens.” (Jayanegara, 2015)
So the evidence suggests that chestnut tannins are the most effective against methane production, IBS, and constipation.
The evidence also suggests that this effect is partially due to the ability of chestnut tannins to bind and precipitate nickel ions.
Tannins from chestnut are available commercially – sold as dietary supplements and for winemaking.

Besides the use of natural chelating agents (such as chestnut tannin), you can restrict nickel availability by simply avoiding it.
This metal is a common component of cookware and utensils.
But nickel‑free versions containing only iron and chromium are also available (e.g. 18/0 and 13/0 stainless steel).
Silver-plated flatware and frying pans are also available – and are safe, antibacterial, and more affordable than commonly believed (such as on eBay).
“…chestnut and sumach tannins, which are hydrolysable tannins, had greater ability to decrease methane concentration than the purified mimosa and quebracho tannins, which are condensed tannins.” (Jayanegara, 2015)
—-Important Message—-
Why super vitamin C is better than any other type of vitamin C
When men take normal vitamin C, it only stays in the bloodstream for a few minutes.
But when men take SUPER vitamin C, it says in the bloodstream for HOURS…
And it penetrates the tissues, the organs, the glands, and even the brain.
So this new SUPER vitamin C delivers more health benefits than regular vitamin C, and it can make men virtually immune to disease.
Here are some examples of how Super C is better than regular Vitamin C:
- Naturally lowers blood pressure, so you can get off Big Pharma treatments and away from all of their dangerous side effects
- Keeps your heart healthy so you don’t have to worry about heart disease…
- Fixes swollen aching joints…with Super C you can start golfing again or run in a 5K race…
- Protects memory and thinking power…now you no longer need to write everything down to remember it…
- Prevents infections and disease — no more colds, flus, or viruses
- Stops cancer in its tracks…potentially life-saving…
Here’s how to get this new SUPER vitamin C today (and it’s free).
———-
