
[cmamad id=”20648″ align=”center” tabid=”display-desktop” mobid=”display-desktop” stg=””]
Humans cannot handle over 300 micrograms of this – but many supplements contain many times that amount…
—–Important Message——
These men locked themselves up for two years – here’s why
These men were isolated for two years conducting an incredible experiment that led to a breakthrough in age research.
And now, a new method has been discovered that does NOT require the hardships they endured…
How?
Well, it’s just one easy thing that I just did with my wife Jodi.
And you could do it today – it only takes minutes!
——–
Check your supplement labels: Is this one of the ingredients?
Today’s newsletter spells out a major problem with a bad supplement that is not only in vitamins but also a lot of “fortified” foods.
Let me start at the beginning.
So even though synthetic molecules are identical to their natural counterparts, folic acid has no natural counterpart.
Folic acid is in multivitamins, B vitamin supplements, and added some foods
Manufacturers prefer this particular folate subtype over freely-available natural forms – not because of stability, but merely on account of its easier chemical synthesis.
Folic acid is simply the cheapest form.
And they justify using that form of vitamin B because some of it is converted into the natural forms within the body.
While this is certainly true, this is not the entire story.
[cmamad id=”20649″ align=”center” tabid=”display-desktop” mobid=”display-desktop” stg=””]
Dihydrofolate reductase – that is the enzyme responsible for this conversion – but dihydrobiopterin reductase can also work.
However, since we haven’t evolved to consume unnatural folic acid at all, we don’t have enzymes to properly deal with it.
Folic acid is the only folate that needs to be reduced twice before it can be used: first into the natural dihydrofolate and then again into tetrahydrofolate.
Since folic acid is unnatural, our bodies had no need to evolve an enzyme to catalyze the first step…
And maybe that is why this first step occurs about 100 times slower than the other, natural one.
Humans cannot handle over 300·μg of folic acid at any given time before it spills over into the bloodstream unconverted.
Now, because of chemical synthesis and food fortification, folic acid is found inside humans for the first time in history.
“Circulating unmetabolized folic acid implies that the body’s capacity to convert folic acid to the metabolically active 5-methyltetrahydrofolate (5-MTFH) has been overwhelmed and that folic acid has passively diffused intact into the circulation. This may have adverse effects…”
Even though folic acid is listed under “vitamins” in food nutrition charts, its only non-precursor function is inhibiting folate-dependent enzymes and brain folate uptake.
Unmetabolized folic acid is consistently linked with reduced brain function, most likely on account of these antagonistic functions.
Although certainly bad, plasma “unmetabolized folic acid” isn’t the whole story:
Only the (6S) forms of folates are active in enzymes that require them…
Yet when folic acid is consumed, the unnatural (6R) enantiomer is also formed:
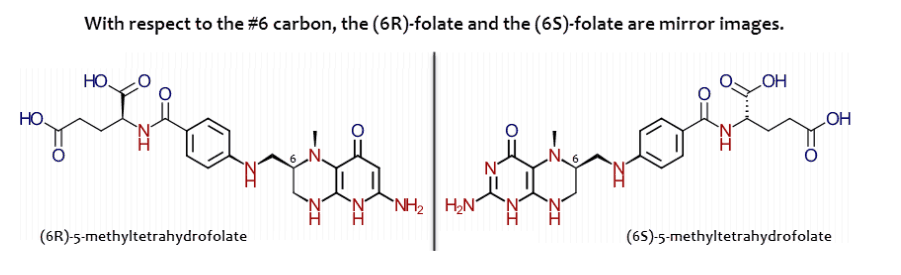
Although our natural enzymes always produce the proper (6S) enantiomers, high doses of folic acid lead to side reactions.
And, just like unmetabolized folic acid, (6R)-folates inhibit enzymes that strictly require the (6S) forms.
Perhaps more importantly: These (6R) derivatives also inhibit brain uptake of the natural (6S)-folates made by plants.
If prolonged, cerebral folate deficiency can lead to demyelination and seizures.
We need the natural (6S)-tetrahydrofolate for DNA synthesis.
The only safe vitamin B9 supplements are those with (6S) stereochemistry.
Both Metafolin® and Quatrefolic® contain (6S) 5-MTFH and are easily available.
Another option is to eat plants for natural (6S)-folates.
And plants leaves also contain vitamin K and Magnesium.
But it’s best to avoid folic acid from B supplements and refined foods.
All of this is well supported by evidence:
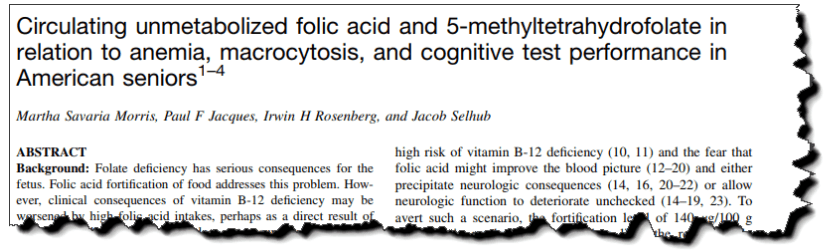
This study compared concentrations of unmetabolized folic acid against a cognitive test in hundreds of subjects.
They gave the subjects the Digit-Symbol Substitution Test (DSST) – it measures attention, learning, memory, and information processing speed.
They found that unmetabolized folic acid in the bloodstream is negatively correlated with test scores for intelligence.
“In the subgroup with low vitamin B 12 status, the mean DSST score for the subjects with detectable circulating unmetabolized folic acid was almost 5 points lower than that of the unexposed subjects…”
As you can see, the effect was more prominent in the low B12 groups.
The enzyme methionine synthase requires cobalamin (vitamin B12) and also (6S)-5-MTHF to convert the more dangerous homocysteine into the safer methionine.
This study implies that unmetabolized folic acid inhibits the enzyme methionine synthase, increasing dangerous homocysteine levels.
Of course, we still need natural folate.
Not surprisingly, this study found the reverse in participants who had more 5-MTHF.
They had an increase in test scores.
We need high amounts of natural folates for proper brain function…
But unnatural folates inhibit natural ones on many levels.
“Results for the 5-MTHF concentration, on the other hand, showed a significant positive association between that exposure and the DSST score.”
All other studies comparing folic acid blood levels or intake have shown the same thing.
These are very consistent observations.
This is also biochemically plausible. Studies done by chemists and pharmacologists have shown that it makes sense for folic acid to inhibit brain folate uptake.
Since folate-dependent enzymes are required for the synthesis of brain DNA, brain myelin, and brain methionine, these findings are not surprising.
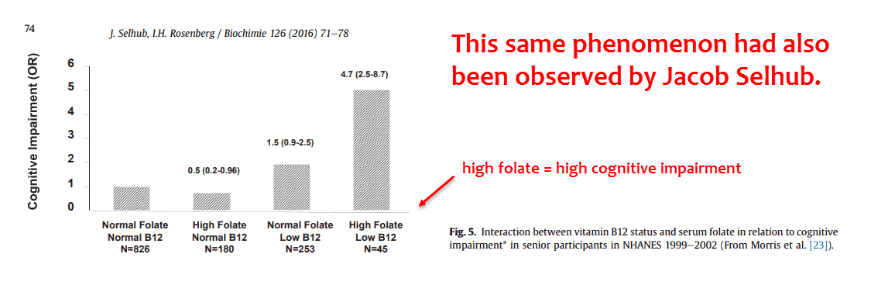
As good as these studies were, they all have one limitation.
None of those studies determined the stereochemistry of the folates.
This study below shows that you can expect a significant amount of (6R)-5-MTFH from taking folic acid – and this is the wrong form and also an enzyme inhibitor.
Inactive (6R)-folates cannot be differentiated from the active (6S)-ones under most analytic techniques…
Therefore, many researchers erroneously take them to be active (6S)-folates.
This is because only (6S)-folates are presumed to occur after taking folic acid…
But the 2004 study below proves that assumption is quite wrong:

This study was the only one to actually measure the stereochemistry of the folates that result from consuming folic acid.
Folates are relevant to cardiologists because their deficiency raises homocysteine – an assumed risk factor for cardiovascular disease.
In the study, they gave patients either folic acid or dl-methyl-tetrahydrofolate, the easier-to-produce form having equal amounts of the (6S)- and (6R)-methylfolates.
Upon ingestion, folic acid will either remain unmetabolized or become: (6S)-folates, (6R)-folates, or a mixture of both.
Since it is commonly assumed that all folic acid is transformed by dihydrofolate reductase in the body, it had always been assumed that all folic acid metabolites are (6S)-folates.
Not only was that assumption wrong, but, actually, more (6R)-folates are actually produced:
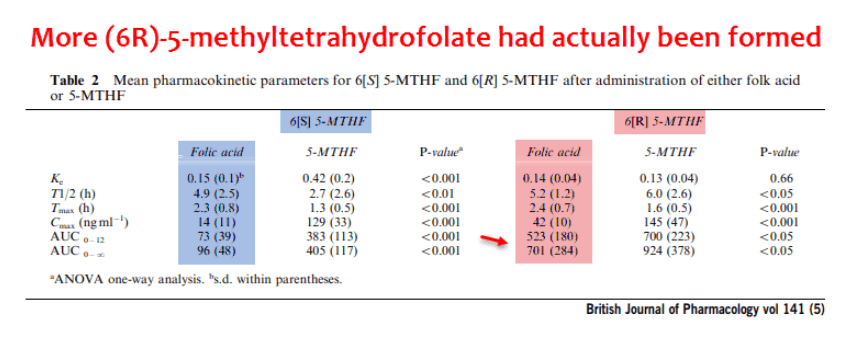
This is hard to reconcile with our natural enzyme system.
Dihydrofolate reductase has been shown to yield exclusively the (6S)-product.
You’d almost be forced to assume that non-enzymatic side reactions are taking place.
And moreover, the amount of active (6S)-folates produced via folic acid ingestion is surprisingly low – almost negligible:
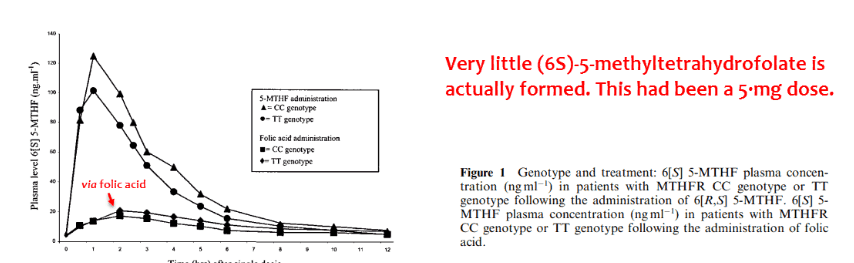
Not only has folic acid been consistently associated with cognitive decline when found in the blood unmetabolized… this form barely yields any active vitamin when it actually is metabolized.
This is unnecessarily dangerous.
Folic acid can inhibit enzymes and lower brain folate uptake while yielding little potential benefit.
The only folate supplements worth buying are exclusively (6S)-folates. Again, you can get those in Metafolin® or Quatrefolic®.
[We are not affiliated with any supplement company.]
Although simply eating green plants and avoiding the unnatural folate forms should be just as good.
—-Important Message—-
Have you tried the New Super Vitamin C?
It’s much more powerful and safe than regular Vitamin C…
- There are so many different vitamin C variations and some of them can be toxic. You will discover the exact amounts and techniques here, including how much vitamin C is too much.
- This new form of Super C stays in your system for days instead of minutes. During that time, germs will just bounce off you. Imagine NEVER having to worry about getting a cold, running a fever, or coughing… And having a virtually steel-hard constitution that NEVER gets sick anymore.
- This new form of Super C produces healthy collagen, the glue that holds cells together and keeps gums healthy. Your dentist will be amazed by your strong and plaque-free teeth.
- Discover how to fix a sore throat or a cold, or even the flu, within a few hours rather than days. This new form of vitamin C works better than any other remedy on the market – because it stays in your system until every trace of disease is neutralized and gone.
- Some vitamin C products contain toxic, harmful ingredients that are cleverly hidden. With this simple vitamin guide, there won’t be any more guessing – you will know exactly what vitamins are safe and which ones have nasty additives and fillers.
Click here to get your own Super-C for FREE
————

http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.975.5917&rep=rep1&type=pdf#page=41
Morris, M. "Circulating unmetabolized folic acid and 5-methyltetrahydrofolate in relation to anemia, macrocytosis, and cognitive test performance in American seniors." The American journal of clinical nutrition (2010)
https://www.researchgate.net/profile/Paul_Jacques/publication/42806517_Circulating_unmetabolized_folic_acid_and_5-methyltetrahydrofolate_in_relation_to_anemia_macrocytosis_and_cognitive_test_performance_in_American_seniors/links/00b7d51e4202c8e429000000/Circulating-unmetabolized-folic-acid-and-5-methyltetrahydrofolate-in-relation-to-anemia-macrocytosis-and-cognitive-test-performance-in-American-seniors.pdf
Obeid, R. "Concentrations of unmetabolized folic acid and primary folate forms in plasma after folic acid treatment in older adults." Metabolism-Clinical and Experimental (2011)
https://www.ncbi.nlm.nih.gov/pubmed/20727555
Xin, W. "Differential stereospecificities and affinities of folate receptor isoforms for folate compounds and antifolates." Biochemical pharmacology (1992)
https://www.sciencedirect.com/science/article/pii/0006295292900892
Selhub, J. "Excessive folic acid intake and relation to adverse health outcome." Biochimie (2016)
https://www.sciencedirect.com/science/article/pii/S0300908416300530
[*] Although the enzyme dihydrobiopterin reductase synthesizes (6R)-tetrahydropiopterins exclusively, these are actually of the same stereochemistry as the (6S)-tetrahydro- folates. The reason they are given different letters is an artifact of the Cahn–Ingold system, which designates stereochemistry based on the priority of connecting bonds. The #6 carbon of biopterins have different priorities as the #6 carbon of folates, and hence get a different number. Under any other system used to designate sterochemistry—such as the d/l and +/− nomenclatures—the natural (6S)-biopterins recieve the same symbol as the natural (6R)-folates.
https://www.medicalnewstoday.com/articles/219853.php
https://www.everydayhealth.com/drugs/folic-acid
https://www.medicinenet.com/folic_acid-oral/article.htm#what_else_should_i_know_about_folic_acid-oral?
Folic acid is also known as Vitamin B-9 is an essential vitamin for the body. Its true importance is its role in the creation of red blood cells. It is an essential part of the B-Complex vitamins. According to the British Diabetic Association, Folic acid has numerous benefits for the body. It also aids the generation of new cells in the body and has been found to boost brain cell growth.
Folic acid is more important for women during pregnancy. It protects the brain development and bones of the infant. Some natural sources of Folic acid are Egg yolk, Cauliflower, Asparagus, Broccoli and Cannage. Folic acid is important not only for women but also for men since it supports some of the most important body processes. It is also known as the DNA creator and is vital for hair growth, skin health and the development of the nails.
Folic acid supplementation is usually recommended by doctors to those who suffer from Anemia. It plays an important role in the regulation of the nervous system and helps in the processing of fats and carbohydrates.
But does supplementation of Folic acid cause any side effects?
The recommended dosage of Folic acid is 400 – 800 mcg orally for adults. Research studies say, since Folic Acid is a soluble Vitamin, any excess will be naturally expelled by the body through urine. Some may complain of an upset stomach. The other side effects include feelings of nausea and the bloating of the stomach. A controlled research study done in 2016 found that some individuals might suffer a loss in appetite from Folic acid supplementation. They complained of trouble in sleeping too.
Some independent research studies have found a probable link of Folic acid consumption with Cancer. It might boost the growth of existing tumors, but research on this topic is still in progress. In addition, since it takes time for the Folic acid molecules to change to an active form and get absorbed in the bloodstream, there may be possible buildups in the blood.
This has been linked to immune system problems and brain disorders in some cases.
There are possible side effects of folic acid interactions if you are on medications. For example, Bile acid related drugs can interact with Folic acid and can cause side effects. In some cases, a Folic acid supplementation overdose can cause conditions like numbness and tingling as well as nausea. Connect with your Doctor if you suffer from any of these side effects. Some people are simply allergic to Folic acid supplementation. In these cases, it is prudent to ask for medical help as soon as possible.
The allergic reaction to Folic acid may include swelling of the face, swelling of the lips and tongue, difficulty in breathing and a bitter taste in the mouth.
Are there any conditions that are caused by a deficiency in Folic acid in the body?
One can suffer from fatigue and weakness when the Folic acid levels in the body fall down. There may be confusion and difficulty with memory retention. Weight loss can occur in some cases while an instance of Appetite loss is also reported.
Pay attention to your doctor's' instructions if you are taking Folic acid supplements. It is prudent to stick to any restrictions on food, beverages or other medications if you are already on a treatment protocol for any other conditions.

Leave a Reply