Our brains get overwhelmed with ammonia and we don’t realize the damage — this spice to the rescue
—-Important Message—-
How to stay rocky after coming — do this

After an incredible romp in the bed, you might be ready to call it a night…
But what if she’s hoping you could pump her juices a little longer?
Well, you could chill out a little… and wait an hour. Or longer…
But she may dry up or lose interest, and the moment may pass you by…
———-
Ammonia builds up in your brain; this spice removes the ammonia
There are very few things that are more dangerous than plasma ammonia.
Homocysteine, serotonin, exorphins, and low folate all take a back seat to ammonia.
Elevated plasma ammonia is the prime cause of hepatic encephalopathy, and what’s mostly responsible for the inebriation seen in long-term alcoholics and liver failure.
And yet, there are so many things in modern society conspiring to cause liver damage:
Hepatitis C, salicylates, aflatoxin, azo dyes, methyl mercury, tetracycline, ochratoxin, aniline, carbon tetrachloride, diethylstilbestrol, ferrous sulfate, mega-dose retinol, and even some mushrooms can induce liver damage.
Some of these likely even work synergistically, and could turn a relatively safe paracetamol dose — for example — into a high-ammonia, low-cognition state.
Because ammonia is a nonpolar gas, it can diffuse everywhere — along with its high volatility, that makes the brain a prime target.
Ammonia (NH3) exists in equilibrium with ammonium (NH4+), a charged species that outnumbers ammonia 71∶1 at bodily pH.
It is actually the ammonium ion that does the most damage, which it can do by displacing potassium — its counterion — from the cell’s interior.
This causes lowering respiration as it’s strongly attracted to the mitochondria, and will also cause swelling and brain edema by effluxing potassium.
NH3 + H2O ⟶ NH4+ + OH−
Even though ammonium is charged, and hence won’t readily enter the brain, it will do so after first losing a proton (H+) and becoming ammonia.
Conversely: ammonia that enters the brain can readily become ammonium by gaining a proton there, causing cerebral swelling which can lead to a coma.
The reason liver damage causes hyperammonemia, of all things, is because this is where 3 essential urea cycle enzymes are exclusively found.
Ornithine transcarbamylase, carbamoyl phosphate synthase, and N-acetylglutamate synthase are only expressed in the liver — and to a much lesser extent the small intestine.
Urea is the primary way in which mammals safely dispose of excess ammonia/ammonium, and with this system impaired, ammonia overload is near-certain to occur.
The only other pathway of ammonia excretion is through the formation of hippuric acid, which is the reason benzoic acid is sometimes given in hyperammonemic emergencies.
Yet most people in modern societies have slightly impaired liver function due to ubiquitous toxins — there could be millions suffering from low-grade impairment on account of this.
Ammonia is like homocysteine, in that the lower the concentration the better, without exception — making its reduction always a good thing.
Molecules like nitric oxide, on the other hand, are dangerous in high amounts, yet still needed for certain things — such as for immunity and blood pressure regulation.
Polyamines and iron are 2 carcinogenic substances we cannot do without, yet ammonia serves absolutely no function in the body besides dose-dependent brain damage.
Yet there are a few reliable ways to greatly enhance ammonia elimination if you suspect overload.
We all know what ammonia smells like, and if your urine smells like THAT you should certainly consider implementing a safe ammonia-lowering agent such as: citrulline, arginine, ornithine, benzoic acid, and/or cinnamaldehyde.
Of these ammonia-lowering agents, there’s good reason to think citrulline is the best one.
While it’s true that arginine and ornithine work in the same way and induce similar ammonia reductions, in some cases arginine is also a substrate of iNOS and ornithine is a substrate of ornithine decarboxylase: the carcinogenic enzyme that synthesizes polyamines.
Ornithine can also be cyclisized into proline, completely annulling its beneficial role in the urea cycle.
And also: arginine must first be converted into urea and ornithine, via arginase, to restart the urea cycle and allow plasma ammonia to be removed.
Arginine can only work after: (1) ornithine becomes citrulline; (2) citrulline becomes arginine; and (3) arginine becomes urea and ornithine again—ad infinitum, over and over again.
This means by taking arginine, you will have to excrete urea first before you can start removing ammonia from the body.
So taking arginine will darken the urine no matter what through urea, so the changes in urine color cannot be taken as an indication of liver function, as when taking ornithine or citrulline.
And yet, citrulline will work synergistically with benzoic acid and cinnamaldehyde.
These 2 molecules: (1) activate the hippuric acid route, (2) are safe to ingest, and (3) would enhance the 2 pathways of ammonia excretion when taken with citrulline.
Cinnamon can contain up to 94 milligrams cinnamaldehyde per gram, which is equivalent to 9.4% by weight.
This is impressive — and so is the fact that 85% of the ingested dose is excreted as hippuric acid, thereby lowering ammonia.
And moreover, cinnamon also inhibits urease: this enzyme is responsible for pointlessly retro-converting urea back into ammonia, negating the entire point of producing it in the first place.
Urease is expressed by bacteria in the intestinal tract, and this enzyme forms ammonia via ingested food.
Cinnamon has been shown to reduce ammonia production by inhibiting urease, and it is safe to ingest in gram-sized amounts.
Cinnamon is the only thing that lowers ammonia in 2 distinct and unrelated ways: It does so first by inhibiting urease, and then again by transforming ammonia into hippuric acid.
Cinnamon is also a powerful antifungal: in Candida and Aspergillus inhibition assays comparing dozens of essential oils, it is nearly always either cinnamon or lemongrass oil that comes out the winner.
This is because these 2 oils have very high concentrations of cinnamaldehyde and citral, molecules that inhibit fungi by mimicking the yeast hormones tyrosol and farnesol.
Yet citrulline is also very important for detoxifying ammonia, an amino acid needed to stimulate the urea cycle.

This is demonstrated most dramatically by its effect in Reye Syndrome: a condition where abberrent liver enzymes and muscle wasting together lead to the most extreme form of hyperammonemia imaginable:
These very high ammonia levels can only be achieved by a massive nitrogen load coupled with liver impairment, as seen in Reye Syndrome.
The ornithine carbamoyltransferase deficiency in the liver, along with muscle catabolism, would be akin to a long-term alcoholic eating 60 eggs per day.
Reye Syndrome is somewhat like a metabolic double-bind, and one that often leads to hyperammonemia, brain ammonium, reduced mitochondrial energy production, cerebral edema, coma, and death — in that order.
In this condition: citrulline and arginine levels quickly plummet as they’re used up and not replenished — the overburdened hippuric acid pathway cannot keep up with the increased nitrogen load. Despite a low- or no-protein diet, ammonia fatally builds up because the muscles generate substantial amounts.
The plasma amino acid analysis highlights this fact, showing citrulline levels at 9% of controls upon admission (blue). Arginine is also low at 48% of the norm (blue), and the massive lysine concentration indicates that muscle is being catabolized (purple).
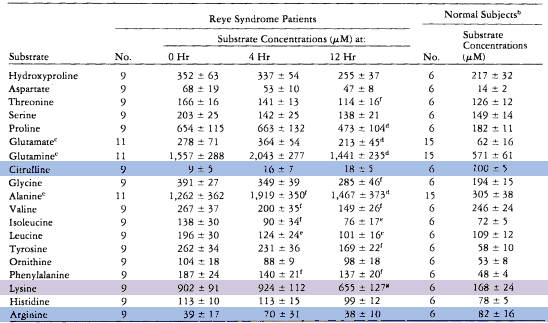
Glutamine levels also increase roughly threefold, and this would represent another excretion pathway if it were more easily eliminated.
The formation of 1 glutamine eliminates 2 ammonia molecules, as it’s formed from glutaric acid, a product of glucose via the Krebs’ Cycle.
On the basis of this chart, you might get the impression that citrulline is needed the most. Arginine is certainly reduced, though not as much, yet ornithine is actually increased.
So even though arginine is the far more common amino acid, and the one more commonly used for hyperammonemia, citrulline appears theoretically superior on all levels.
Only citrulline, not arginine or ornithine, completely bypasses ornithine carbamoyltransferase and carbamoyl phosphate synthase.
Since these 2 urea cycle enzymes are expressed only in the liver, they are both more characteristic of liver failure than the others.
The 3 enzymes that detoxify ammonia by transforming citrulline into arginine — i.e., argininosuccinate synthetase, argininosuccinate lyase, and arginase — are found nearly everywhere in the body and are less significantly inhibited upon liver damage.
‘One of the salient metabolic characteristics of Reye syndrome is a marked catabolic state in which nitrogenous products overburden the body’s mechanisms for metabolizing ammonia. At the same time, there is evidence of decreased activity of the urea cycle in the liver, the principal means for detoxifying ammonia.’ ―DeLong
So based on theoretical considerations, Dr. Robert DeLong kept citrulline on standby and waited for cases of Reye Syndrome to come in.
Over a period of a few years, he successfully used citrulline to save patients from ammonia-induced brain death.
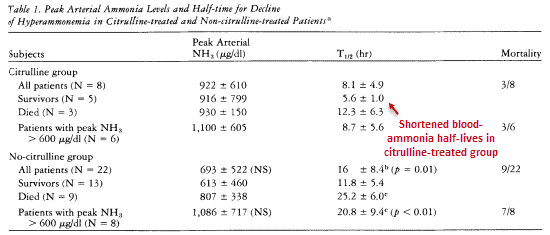
He even took frequent measurements of the plasma, recording ammonia levels multiple times per hour. He plotted the reduction of plasma ammonia vs time, which he then used to calculate the duration until ammonia became ½ the average value.
The average time it took to hit the median ammonia concentration of average people was reduced from 20.8 hours in the controls to 8.7 hours in his citrulline-treated patients.
This occurred despite the citrulline group starting with much higher plasma ammonia concentrations:
While it’s true that 3 patients died in the citrulline-treated group, at least 2 of these had nothing at all to do with its efficacy.
1 patient was actually brain-dead on arrival and was treated anyway, and 1 other was accidentally killed by a nurse at night while fumbling with the endotracheal tube.
It is likely that the third case could not have been saved by anything.
‘…the third hospital day she was rousing from coma and metabolic abnormalities had subsided, but during the night she suddenly died. Though the cause was uncertain, it was thought to be due to an accident with the endotracheal tube; clearly, it was not due to cerebral swelling.’ ―DeLong
So clearly: citrulline works very effectively even in the most severe cases, such as Reye’s Syndrome patients with plasma ammonia concentrations over 900 μg/dl.
This was achieved at only 35 to 100 mg/kg orally every 4 hours, certainly impressive considering the doses of arginine shown to be not nearly as effective.
Doctors using arginine have used 348 to 697 mg/kg arginine intravenously, and this wasn’t capable of achieving the same effect.
Citrulline can work in cases where arginine cannot, such as with a low activity of ornithine carbamoyltransferase — a liver-specific enzyme.
And citrulline is not a substrate of nitric oxide synthase, but a product of it.
This means that much of the efficacy of arginine in Reye’s Syndrome could in fact be due to its prior transformation into citrulline, via iNOS, creating nitric oxide in the process.
This is supported by the fact that ornithine carbamoyltransferase is the 1 enzyme failure almost always found in Reye’s Syndrome, and that arginine cannot detoxify ammonia without going through this.
Citrulline deftly bypasses this enzyme, so is better than arginine for this reason and also many others.
‘…these findings indicate an accelerated normalization of ammonia-to-urea conversion in the citrulline-treated patients.’ ―DeLong
And nitric oxide has been implicated in some of the brain damage found in hyperammonemia.
This means that when compared to citrulline, arginine could be seen as potentially damaging, considering the nitric oxide formed.
And as a precursor to the polyamines, ornithine probably isn’t something you’d want to be taking every day.
So certainly, the combination of cinnamon and citrulline seem the best approach for general ammonia reduction.
Although many brands of soda do have benzoic acid, drinking too much of this could potentially cause other problems.
Yet during an emergency, soda could probably be seen as a good thing — both for the benzoic acid and the glucose.
Glucose is given in muscle-wasting situations because insulin is anabolic. Also, sugar metabolites formed from it, via the Krebs’ Cycle, have their own minor roles in detoxifying ammonia.
Glutaric acid can safely hold 2 ammonia molecules as it becomes glutamine, and succinate is ultimately needed to regenerate citrulline.
‘This effect is accentuated if only those cases with peak ammonia greater than 600 μg/dl are compared; in these patients mean T1/2 for decline of hyperammonemia in the citrulline-treated group was 8.7 hours as compared to 20.8 hours for the non-citrulline-treated group.’ ―DeLong
Cinnamon certainly goes well with coffee, and citrulline is best taken with meals.
Caution: only use Ceylon cinnamon as other cinnamon can be toxic!!
And be careful of supplementing with citrulline or arginine — getting it in foods is best.
Ammonia is even worse for the brain than homocysteine and serotonin, and ought to be kept low, unless you’d like to start mumbling like the town drunk.
—-Important Message—-
Men: here’s how to activate the neurotrophic factor and increase pleasure 70%

This reverses decades of low penile sensitivity, providing erections like it was when you were a young man…and making pleasure exquisite…
Remember: men can lose 70-80% of their penile sensitivity by the time they’re 50…
But with the neurotrophic factor, you restore sensation and sensitivity back into your member…
…and you can actually INCREASE your pleasure threshold, so you feel more than you ever thought possible…
Perfect for these situations:
- Men who have experienced difficulty getting “rocky”
- Men who are going soft during intercourse
- Men who grind away and can’t come at all
Once you focus on your lack of penile feeling, you are suddenly aware that this has been a problem forever!
It’s like realizing you need glasses — everything seems fine until you put them on for the first time and realize how much BETTER things can be…
Discover how to activate the neurotrophic factor for free right now
———-
