
[cmamad id=”20036″ align=”center” tabid=”display-desktop” mobid=”display-desktop” stg=””]
Just take an extra dose of this vitamin and your red meat will deliver double the nutrients…
—–Important Message—–
This new method can remove 3 inches from your belly in 24 hours, AND give you better erections…
This method works by removing dangerous blood fat, called free fatty acids.
When blood fat no longer circulates, the fat cells that store fat are kick-started into burning fat that’s already IN the fat cells.
Fat cells literally shrink. Your belly is noticeably flatter. And your male member begins perking up because the male member HATES blood fat.
Discover the method that allows you to burn belly fat and boost your erections…

—————–
This Is How You Should Be Eating Red Meat
In the 1950s, Ancel Keys implicated saturated fat and cholesterol as being responsible for cardiovascular disease.
This conclusion has turned out to be severely wrong (though many people and some of the media still ascribe to his hypothesis).
But there is still one association with red meat that persists: Red meat has been consistently associated with colon cancer for nearly a century.
This is unfortunate because beef has the best fatty acid profile – so there has to be a way to circumvent this…
Although eating red meat is not exactly a death sentence, risk ratios around 2.0 are consistent findings:
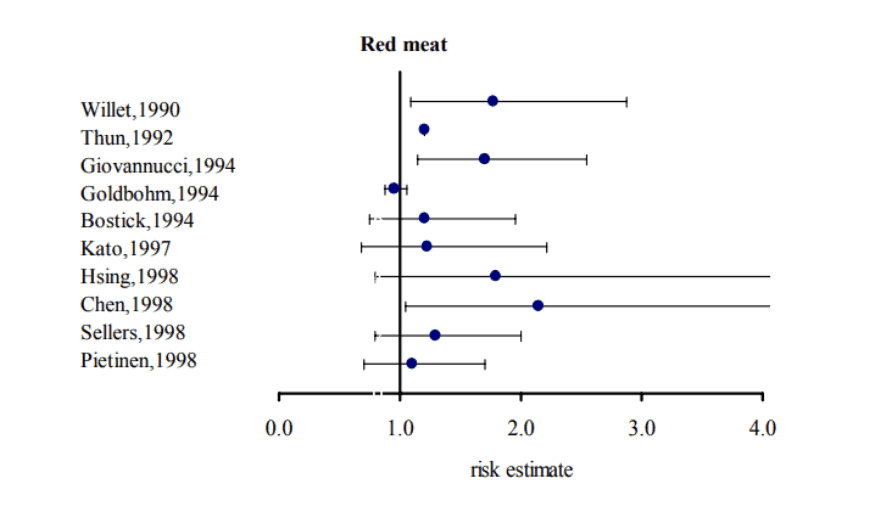
Lucky for us, scientists now know the exact reason for this higher risk rate.
With the proper food combining, we can entirely negate this risk level and eat beef safely.
For decades, some scientists have known exactly why red meat is safe.
Also persisting for generations, we have the notion that “white meat” is not associated with colon cancer in any way:
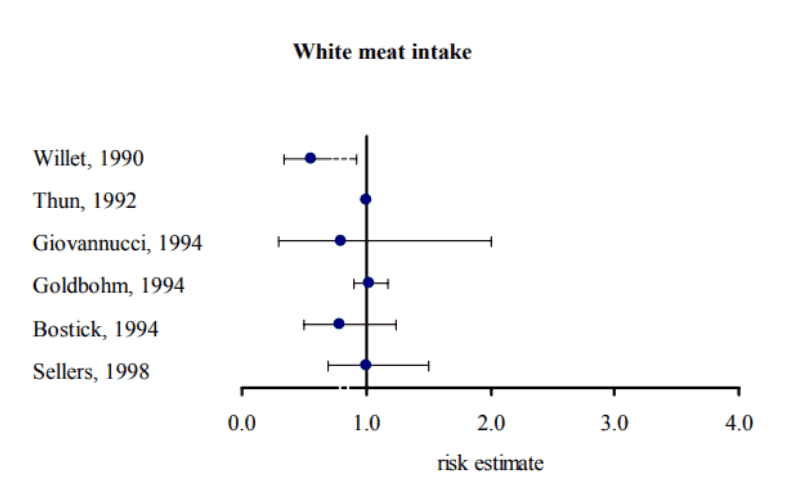
So what is the difference?
Both red and white meat have similar amounts of protein, vitamins, minerals, steroids, etc.
[cmamad id=”20037″ align=”center” tabid=”display-desktop” mobid=”display-desktop” stg=””]
So the difference certainly cannot be the fatty acids.
The difference is, of course, the color.
The heme-iron complex is what gives red meat its color…
(Heme iron is the type of iron found in blood and muscle.)
This type of iron is found in greater concentrations in red meat than in white meat:
“…the amount of heme in red meat is much higher than in white meat, for instance: 0.25–0.5 µmol/g for beef versus 0.02–0.05 µmol/g for chicken.”
Heme iron is roughly ten times higher in beef, horse, and lamb than it is in turkey, chicken, and pork.
It’s not just “total iron” that matters either, even though this is also 10 times higher in red meat.
The idea that free iron can induce cancer just as well as heme iron – well, experiments prove that this is not the case:

In 2003, twenty-one healthy males serving as their own controls ate different diets consisting of nearly every kind of food…
Red meat, eggs, white meat, peanuts, liver pâté, kidney beans, green lentils, cream, glucose polymers, low-fat cheese, blood sausage, and more.
These different diets were isocaloric (matched for calories), and often normalized for total iron and total protein.
In each case, the researchers determined the amount of nitrosamines formed.
Nitrosamines are unstable, reactive, and potent carcinogens that appear to work by permeating the cell’s nucleus and directly modifying DNA.
The researchers found that the total amount of nitrosamines formed is a direct function of red meat intake – exclusively the red meat, not the total protein (which was held constant):
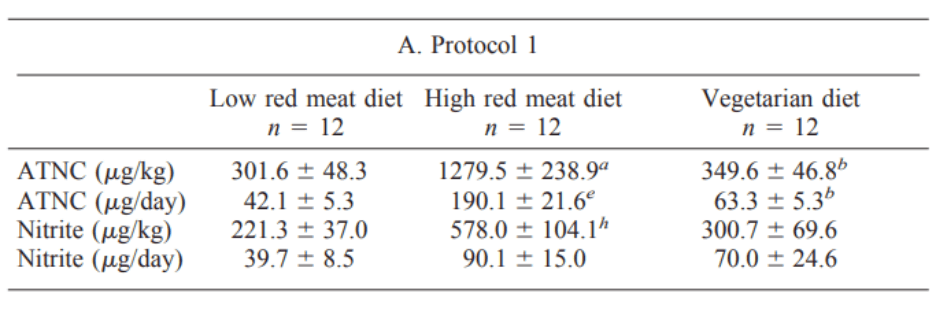
This group’s earlier study comparing white to red meat showed the very same trend, completely exonerating chicken and pork from nitrosamine formation.
Even though protein contains essentially all of our food’s amino groups, nitrosamine formation will be the same regardless of the source of the protein.
Actually, it seems that heme iron is mostly responsible for nitrosamine formation… Inorganic iron will not do this:
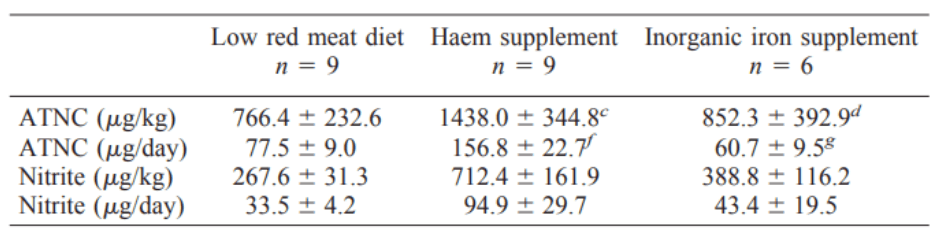
So what is so special about heme and nitrosamines?
Heme’s central iron atom has a propensity for binding gases such as oxygen and carbon monoxide.
But it can also bind ozone, cyanide, and even nitric oxide.
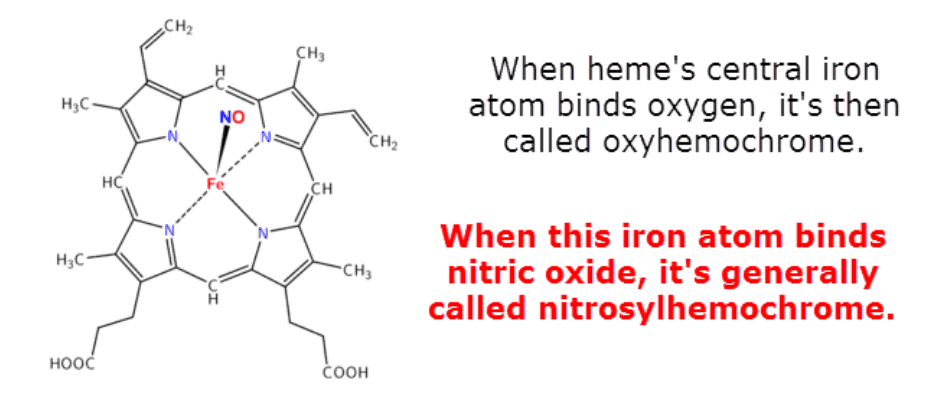
This is its main function within the body.
Heme normally carries oxygen – as hemoglobin – throughout the bloodstream to the body’s periphery.
Carbon monoxide causes asphyxia by inhibiting this process.
This is also partially how nitric oxide exerts its mutagenic effects.
But it’s not always nitric oxide per se that is bound.
Depending on the electron configuration, the bound species can leave as either nitric oxide (Ṅ=O) or as nitrosonium (N≡O:+).
The difference there is only one electron.
Although excessive nitric oxide can be considered toxic, it’s not actually the worst reactive nitrogen species…
Nitrogen dioxide, peroxynitrite, and nitrosonium are all more reactive.
The nitrosonium ion (O≡N+) is what forms nitrosamines, so it’s pretty much synonymous with cancer.
The dependence on electrons for nitrosamine formation actually suggests a partial antidote against their formation:

For decades, studies have been showing that vitamin C protects against cardiovascular disease and cancer of all types.
Of course, some of this depends on collagen. The synthesis of collagen is catalyzed by vitamin C.
A low collagen turnover will lower tissue integrity, and that facilitates the invasion of cancer cells.
But here is what vitamin C does to heme…
Vitamin C donates electrons to heme’s extensive ring complex, and that leads to more nitric oxide than nitrosonium being released.
“If either ascorbate or cysteine was added to the solutions, nitrosyl-metmyoglobin was reduced to nitrosyl-myoglobin … higher concentrations could not be studied because of the evolution of nitric oxide.”
So it is largely the redox status of the environment that dictates whether the dangerous nitrosonium ion is released or the less carcinogenic nitric oxide (Fox, 1963).
(Redox is a process in which one substance or molecule is reduced and another oxidized.)
Only nitrosonium forms nitrosamines.
Epidemiological study results back up the chemical observations…
But we should consume enough vitamin C regardless.
Since vitamin C also lowers nitrosamine formation, it could be a good idea to take vitamin C when eating red meat.
And there’s also inositol hexaphosphate (phytic acid).
This is a powerful iron chelator in whole grains, nuts, and seeds. It actually binds the heme complex directly.
Although it hasn’t been shown to sequester or commandeer heme’s central iron atom, it can at least break two histidine bonds and force it out of plane.
“…where inositol hexaphosphate leads to the cleavage of the proximal histidine to ferrous oxide bonds, there is movement of the iron atom out of the porphyrin plane toward the nitric oxide ligand…”
“…and a change in iron-nitrogen-oxygen bond angles which are accompanied by disturbance of the ‘normal’ interactions of the nitric oxide with adjacent distal histidine and, possibly, other amino acid residues.”
You might be forced to think that this would more or less disable it…
And this idea gains support by considering that whole-grain bread has often been found associated with a lower colon cancer incidence.
Whole grain bread does contain phytic acid…but it also has gluten.
However, we can circumvent many of the negative effects of wheat by buying sourdough bread.
The specific bacteria used in the production of sourdough largely hydrolyzes the problematic gluten proteins.
And choosing meat with low nitrites should be helpful.
The food industry adds nitrites to meat more as a coloring agent than as a bacterial defense.
Nitrite ions (O=N–O−) can also add to heme, shifting its color spectrum to bright red.
But they also pre-load it with bound nitric oxide (Ṅ=O) for nitrosamine production.
But even “nitrite-free” meat often has added nitrites, sometimes simply labeled “spices” or “flavorings” – celery powder is naturally high in it…
While this stuff is actually a flavoring, they add it to give the meat a nicer color – to enhance sales.
There is nothing practical about added nitrites in any form.
Fully-oxidized heme is brown, but that is not consumer-friendly.
—–Important Message—–
“I am no longer a type 2 diabetic”

Click here to try my diabetes-reversing program for just $1 today…
—————–


Leave a Reply